‘Heart on a chip’ reduces time and cost in drug testing for safety and efficacy
March 10, 2015

The “heart-on-a-chip” developed at UC Berkeley houses human heart tissue derived from adult stem cells. Drugs to be tested are inserted into the tube on top, which connects to simulated blood vessels. The system could one day replace animal models for drug safety screening. (credit: Anurag Mathur, Healy Lab)
A UC Berkeley research team led by bioengineering professor Kevin Healy has developed a network of pulsating cardiac muscle cells that models human heart tissue.
They have also demonstrated the viability of this system as a drug-screening tool by testing it with cardiovascular medications.
This “organ-on-a-chip,” housed in an inch-long silicone (a rubberlike material) device, represents a major step forward in the development of accurate, faster methods of testing for drug toxicity, the researchers say.
Replacing animal testing
“Ultimately, these chips could replace the use of animals to screen drugs for safety and efficacy,” said Healy.
The study authors noted that nonhuman animal models have a high failure rate associated in predicting human reactions to new drugs.
Much of this is due to fundamental differences in biology between species, the researchers explained. For instance, the ion channels through which heart cells conduct electrical currents can vary in both number and type between humans and other animals.
“Many cardiovascular drugs target those channels, so these differences often result in inefficient and costly experiments that do not provide accurate answers about the toxicity of a drug in humans,” said Healy.
“It takes about $5 billion on average to develop a drug, and 60 percent of that figure comes from upfront costs in the research and development phase. Using a well-designed model of a human organ could significantly cut the cost and time of bringing a new drug to market.”
How the heart-on-a-chip works
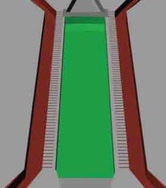
The cardiac microphysiological system mimics the essential structure and functions of the heart. The simulated blood vessels (red-brown) supply the drug to be tested as well as nutrients (glucose, oxygen, etc.) via microchannels that simulate the endothelial barrier (horizontal gray lines) to simulated heart cells (green). (credit: Anurag Mathur et al./Scientific Reports)
The heart cells were derived from human-induced pluripotent stem cells, the adult stem cells that can be coaxed to become many different types of tissue.
The researchers designed their cardiac microphysiological system, or heart-on-a-chip, so that its 3D structure would be comparable to the geometry and spacing of connective tissue fiber in a human heart.
They added the differentiated human heart cells into the loading area; this confined geometry helps align the cells in multiple layers and in a single direction.
Microfluidic channels on either side of the cell area serve as models for blood vessels, mimicking the exchange by diffusion of nutrients and drugs with human tissue. In the future, this setup could also allow researchers to monitor the removal of metabolic waste products from the cells.
It’s alive! (almost)
“This system is not a simple cell culture where tissue is being bathed in a static bath of liquid,” said study lead author Anurag Mathur, a postdoctoral scholar in Healy’s lab and a California Institute for Regenerative Medicine fellow. “We designed this system so that it is dynamic; it replicates how tissue in our bodies actually gets exposed to nutrients and drugs.”

Silicon wafers like the one shown here are used to create silicone-based “organ-on-a-chip” devices (via a photolithography process) to model human tissue (credit: Anurag Mathur, Healy Lab)
Within 24 hours after the heart cells were loaded into the chamber, they began beating on their own at a normal physiological rate of 55 to 80 beats per minute.
The researchers tested the system by monitoring the heart tissue’s beat rate for four well-known cardiovascular drugs: isoproterenol, E-4031, verapamil and metoprolol.
The baseline beat rate for the heart tissue consistently fell within 55 to 80 beats per minute, a range considered normal for adult humans.
After exposure to the drugs the results were predictable. For example, after half an hour of exposure to isoproterenol, a drug used to treat bradycardia (slow heart rate), the beat rate of the heart tissue increased from 55 to 124 beats per minute.
Modeling diseases and drug reactions
The researchers noted that their heart-on-a-chip could be adapted to model human genetic diseases or to screen for an individual’s reaction to drugs. They are also studying whether the system could be used to model multi-organ interactions. A standard tissue culture plate could potentially feature hundreds of microphysiological cell culture systems.
“Linking heart and liver tissue would allow us to determine whether a drug that initially works fine in the heart might later be metabolized by the liver in a way that would be toxic,” said Healy.
The engineered heart tissue remained viable and functional over multiple weeks. Given that time, it could be used to test various drugs, Healy said.
The system could also accommodate “hundreds of cell culture channels and thus enable medium throughput screening,””Furthermore, the combination of the MPS with the isogenic GCaMP6f reporter cell line and automatized video analysis software enables the high throughput analysis of mechanical and electrophysiological properties of the microtissue.”
The open-access study was published Monday, March 9 in the journal Scientific Reports. The project is funded through the Tissue Chip for Drug Screening Initiative, an interagency collaboration launched by the National Institutes of Health to develop 3-D human tissue chips that model the structure and function of human organs.
UC Berkeley | Heart on a Chip
This video shows human heart tissue, derived from adult stem cells, before and after exposure to isoproterenol, a drug used to treat bradycardia (slow heart rate) and other heart problems. The beat rate visibly increased after 30 minutes of exposure to the drug. (credit: Anurag Mathur, PhD, Healy Lab)
Abstract of Human iPSC-based Cardiac Microphysiological System For Drug Screening Applications
Drug discovery and development are hampered by high failure rates attributed to the reliance on non-human animal models employed during safety and efficacy testing. A fundamental problem in this inefficient process is that non-human animal models cannot adequately represent human biology. Thus, there is an urgent need for high-content in vitro systems that can better predict drug-induced toxicity. Systems that predict cardiotoxicity are of uppermost significance, as approximately one third of safety-based pharmaceutical withdrawals are due to cardiotoxicty. Here, we present a cardiac microphysiological system (MPS) with the attributes required for an ideal in vitro system to predict cardiotoxicity: i) cells with a human genetic background; ii) physiologically relevant tissue structure (e.g. aligned cells); iii) computationally predictable perfusion mimicking human vasculature; and, iv) multiple modes of analysis (e.g. biological, electrophysiological, and physiological). Our MPS is able to keep human induced pluripotent stem cell derived cardiac tissue viable and functional over multiple weeks. Pharmacological studies using the cardiac MPS show half maximal inhibitory/effective concentration values (IC50/EC50) that are more consistent with the data on tissue scale references compared to cellular scale studies. We anticipate the widespread adoption of MPSs for drug screening and disease modeling.