Hijacking the bacterial ‘communication system’ to tell cancer cells to stop spreading — or even die
October 3, 2014
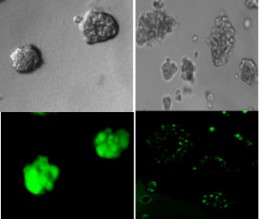
Cancer cells on the left are pre-molecule treatment. The cells on the right are after the treatment and are dead (credit: S. Kumar)
A molecule used as a bacteria communication system can be hijacked and used to prevent cancer cells from spreading — or even to die on command, University of Missouri researchers have discovered.
“During an infection, bacteria release molecules which allow them to ‘talk’ to each other,” explained Senthil Kumar, an assistant research professor and assistant director of the Comparative Oncology and Epigenetics Laboratory at the MU College of Veterinary Medicine, the lead author of the study.
“Depending on the type of molecule released, the signal will tell other bacteria to multiply, escape the immune system, or even stop spreading. We found that if we introduce the ‘stop spreading’ bacteria molecule to cancer cells, those cells will not only stop spreading; they will begin to die as well.”
In the study, published in PLOS ONE, the researchers treated human pancreatic cancer cells grown in culture with bacterial communication molecules called ODDHSL. After the treatment, the pancreatic cancer cells stopped multiplying, failed to migrate, and began to die.
“We used pancreatic cancer cells because those are the most robust, aggressive and hard-to-kill cancer cells that can occur in the human body,” Kumar said.
“Because this treatment shows promise in such an aggressive cancer like pancreatic cancer, we believe it could be used on other types of cancer cells and our lab is in the process of testing this treatment in other types of cancer.”
“At this time, we only are able to treat cancer cells with this molecule in a laboratory setting,” Kumar said. “We are working on a better method which will allow us to treat animals with cancer to see if this therapy is truly effective. The early-stage results of this research are promising. If additional studies, including animal studies, are successful then the next step would be translating this application into clinics.”
Abstract of Bacterial Quorum Sensing Molecule N-3-Oxo-Dodecanoyl-L-Homoserine Lactone Causes Direct Cytotoxicity and Reduced Cell Motility in Human Pancreatic Carcinoma Cells
In spite of chemotherapeutic and surgical advances, pancreatic cancer continues to have a dismal prognosis. Metastasis due to tumor cell migration remains the most critical challenge in treating pancreatic cancer, and conventional chemotherapy is rarely curative. In the quest for more novel molecules to fight this disease, we tested the hypothesis that the Pseudomonas aeruginosa quorum sensing signal molecule N-3-oxo-dodecanoyl-L-homoserine lactone (O-DDHSL) would be cytotoxic to and reduce mobility of pancreatic carcinoma cells (Panc-1 and Aspc-1). Results showed a decrease in cell viability from apoptosis, diminished colony formation, and inhibition of migration of the evaluated pancreatic carcinoma cell lines. Also, cell viability decreased in the presence of O-DDHSL when cells were grown in matrigel basement membrane matrix. While messenger RNA for IQGAP-1 decreased in Panc-1 and HPDE cells upon exposure to O-DDHSL, no change was observed in Aspc-1 cells. Cofilin mRNA expression was found to be increased in both HPDE and Panc-1 cells with marginal decrease in Aspc-1 cells. RhoC, a Rho-family GTPase involved in cell motility, increased in the presence of O-DDHSL, suggesting a possible compensatory response to alteration in other migration associated genes. Our results indicate that O-DDHSL could be an effective biomolecule in eukaryotic systems with multimodal function for essential molecular targeting in pancreatic cancer.