How memory and thought alter the meaning of odors
March 4, 2014
Odors have a way of connecting us with moments buried deep in our past. But researchers have long wondered how the process works in reverse: how do our memories shape the way sensory information is collected?
In work published in Nature Neuroscience, scientists from Cold Spring Harbor Laboratory (CSHL) demonstrate for the first time a way to observe this process in awake animals.
The team, led by Assistant Professor Stephen Shea, was able to measure the activity of a group of inhibitory neurons that links the odor-sensing area of the brain with brain areas responsible for thought and cognition. This connection provides feedback so that memories and experiences can alter the way smells are interpreted.
The inhibitory neurons that forge the link are known as granule cells. They are found in the core of the olfactory bulb, the area of the mouse brain responsible for receiving odor information from the nose. Granule cells in the olfactory bulb receive inputs from areas deep within the brain involved in memory formation and cognition.
Granule cells relay the information they receive from neurons involved in memory and cognition back to the olfactory bulb. There, the granule cells inhibit the neurons that receive sensory inputs. In this way, “the granule cells provide a way for the brain to ‘talk’ to the sensory information as it comes in,” explains Shea. “You can think of these cells as conduits which allow experiences to shape incoming data.”
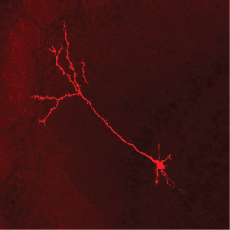
CSHL researchers have engineered a system to observe how experiences shape the way sensory input is collected. For the first time, they were able to measure the activity of inhibitory neurons called granule cells (shown here) that link the sense of smell with memory and cognition in awake animals, offering insight into how our expectations influence the way we perceive the world around us. ( Credit: Stephen Shea, Cold Spring Harbor Laboratory)
Why might an animal want to inhibit or block out specific parts of a stimulus, like an odor? Every scent is made up of hundreds of different chemicals, and “granule cells might help animals to emphasize the important components of complex mixtures,” says Shea.
For example, an animal might have learned through experience to associate a particular scent, such as a predator’s urine, with danger. But each encounter with the smell is likely to be different. Maybe it is mixed with the smell of pine on one occasion and seawater on another. Granule cells provide the brain with an opportunity to filter away the less important odors and to focus sensory neurons only on the salient part of the stimulus.
Now that it is possible to measure the activity of granule cells in awake animals, Shea and his team are eager to look at how sensory information changes when the expectations and memories associated with an odor change. “The interplay between a stimulus and our expectations is truly the merger of ourselves with the world. It exciting to see just how the brain mediates that interaction,” says Shea.
This work was supported by the Klingenstein fellowship and a fellowship from the Natural Sciences and Engineering Research Council of Canada.
Abstract of Nature Neuroscience paper
Olfactory representations are shaped by brain state and respiration. The interaction and circuit substrates of these influences are unclear. Granule cells (GCs) in the main olfactory bulb (MOB) are presumed to sculpt activity reaching the cortex via inhibition of mitral/tufted cells (MTs). GCs potentially make ensemble activity more sparse by facilitating lateral inhibition among MTs and/or enforce temporally precise activity locked to breathing. Yet the selectivity and temporal structure of wakeful GC activity are unknown. We recorded GCs in the MOB of anesthetized and awake mice and identified state-dependent features of odor coding and temporal patterning. Under anesthesia, GCs were sparsely active and strongly and synchronously coupled to respiration. Upon waking, GCs desynchronized, broadened their tuning and largely fired independently from respiration. Thus, during wakefulness, GCs exhibited stronger odor responses with less temporal structure. We propose that during wakefulness GCs may shape MT odor responses through broadened lateral interactions rather than respiratory synchronization.
