How to detect early signs of Alzheimer’s with a simple eye exam before symptoms appear
July 12, 2016
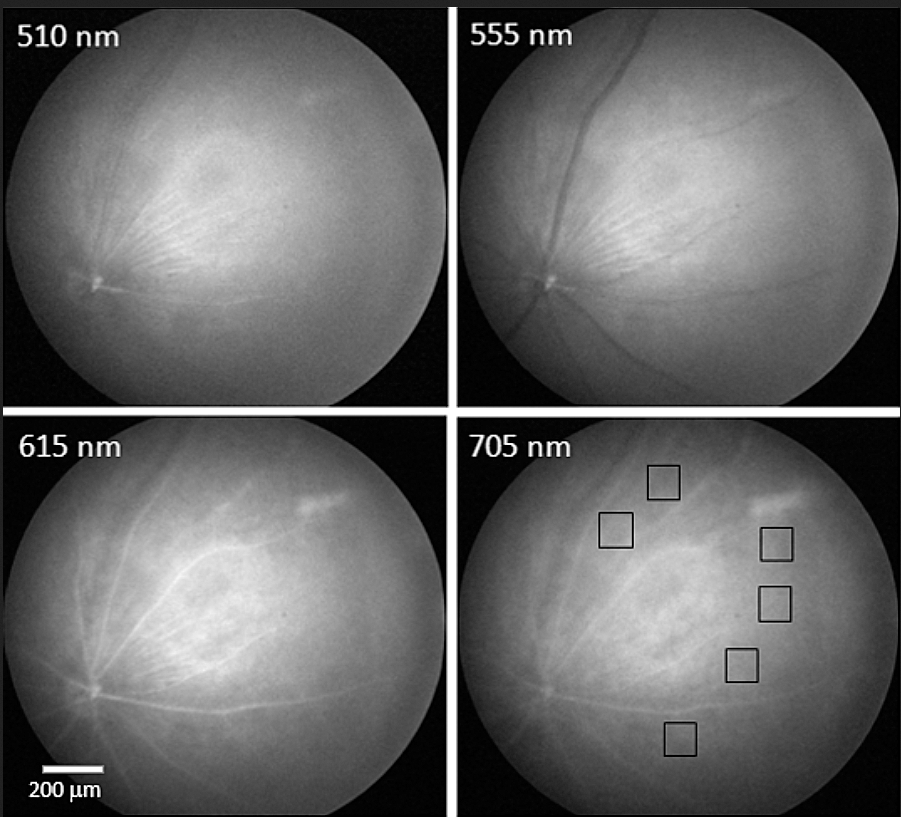
Monochromatic images of normal mouse retina at four wavelengths from blue (upper left) to red (lower right). Boxes in lower right show locations typical of amyloid (a substance found in the brain associated with Alzheimer’s). (credit: Swati S. More et al./IOVS)
University of Minnesota (UMN) scientists and associates have developed new technology that can detect signs of Alzheimer’s before the onset of symptoms — early enough to give drugs a chance to work — in mice and humans by simply examining the back of their eyes.
Looking at Alzheimer’s effects through the eye is a key advantage of the new technology. “The retina of the eye is not just ‘connected’ to the brain — it is part of the central nervous system,” said Swati More, PhD, of the Center for Drug Design at UMN, co-author of a paper recently published in Investigative Ophthalmology & Visual Science (IOVS).
The brain and retina undergo similar changes due to Alzheimer’s disease, he said, but “unlike the brain, the retina is easily accessible to us. We saw changes in the retinas of Alzheimer’s mice before the typical age at which neurological signs are observed.”
Human clinical trials are set to start in July to test the technology in humans who are 40–75 years old (for more information on participating in the clinical trial, you can visit the trial website).
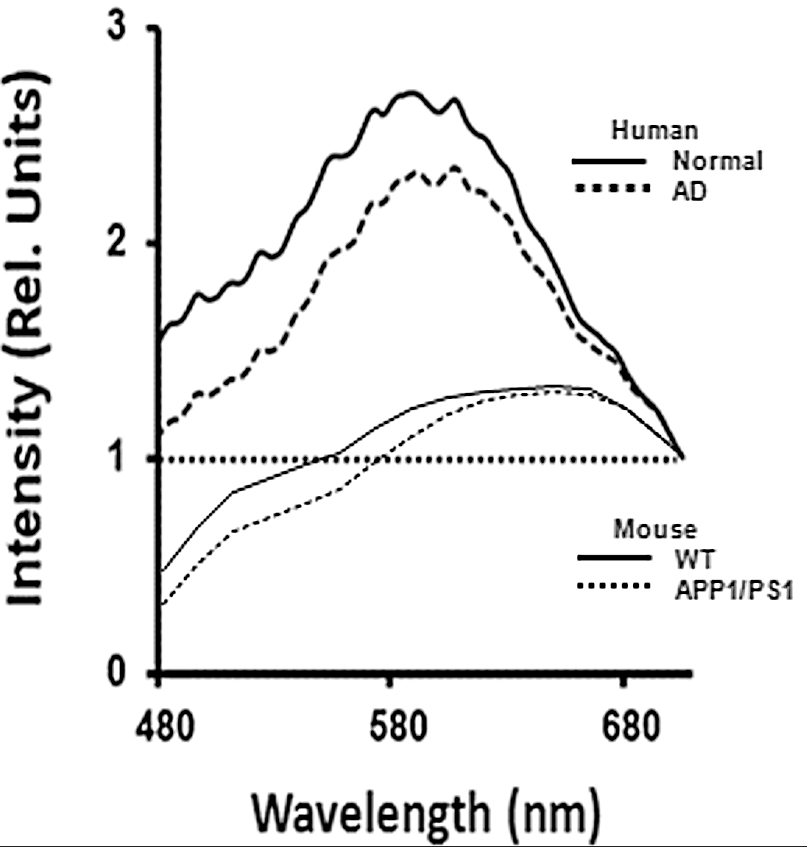
Optical spectra recorded from human and mouse retina samples. Upper two lines show the intensity of light for different wavelengths of light from red (right side) to blue (left side) for a normal human retina (heavy line) and for an Alzheimer’s (AD) patients retina. Comparable mouse plots, shown below (WT = normal mouse; APP1/PS1 = Alzheimer’s model mouse), show a similar pattern. (credit: Swati S. More et al./IOVS)
Early detection of Alzheimer’s is critical for two reasons. “First, effective treatments need to be administered well before patients show actual neurological signs,” said co-author Robert Vince, PhD, of the Center for Drug Design at the University of Minnesota (UMN). “Second, since there are no available early detection techniques, drugs currently cannot be tested to determine if they are effective against early Alzheimer’s disease. An early diagnostic tool like ours could help the development of drugs as well.”
Abstract of Early Detection of Amyloidopathy in Alzheimer’s Mice by Hyperspectral Endoscopy
Purpose: To describe a spectral imaging system for small animal studies based on noninvasive endoscopy of the retina, and to present time-resolved spectral changes from live Alzheimer’s mice prior to cognitive decline, corroborating our previous in vitro findings.
Methods: Topical endoscope fundus imaging was modified to use a machine vision camera and tunable wavelength system for acquiring monochromatic images across the visible to near-infrared spectral range. Alzheimer’s APP/PS1 mice and age-matched, wild-type mice were imaged monthly from months 3 through 8 to assess changes in the fundus reflection spectrum. Optical changes were fit to Rayleigh light scatter models as measures of amyloid aggregation.
Results: Good quality spectral images of the central retina were obtained. Short-wavelength reflectance from Alzheimer’s mice retinae showed significant reduction over time compared to wild-type mice. Optical changes were consistent with an increase in Rayleigh light scattering in neural retina due to soluble Aβ1–42aggregates. The changes in light scatter showed a monotonic increase in soluble amyloid aggregates over a 6-month period, with significant build up occurring at 7 months.
Conclusions: Hyperspectral imaging technique can be brought inexpensively to the study of retinal changes caused by Alzheimer’s disease progression in live small animals. A similar previous finding of reduction in the light reflection over a range of wavelengths in isolated Alzheimer’s mice retinae, was reproducible in the living Alzheimer’s mice. The technique presented here has a potential for development as an early Alzheimer’s retinal diagnostic test in humans, which will support the treatment outcome.