How to form the world’s smallest self-assembling nanowires — just 3 atoms wide
December 30, 2016
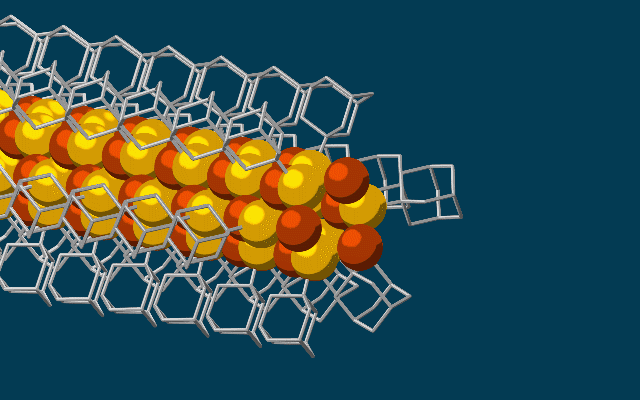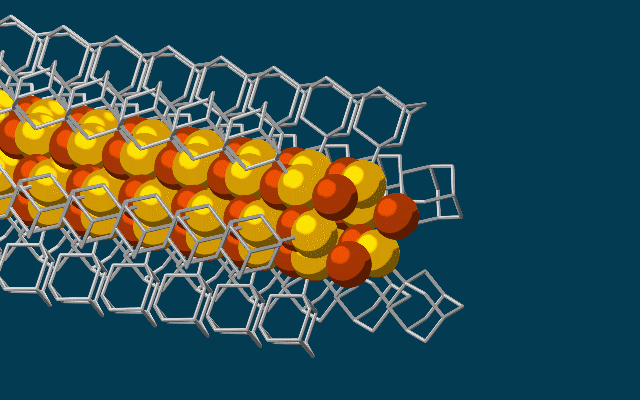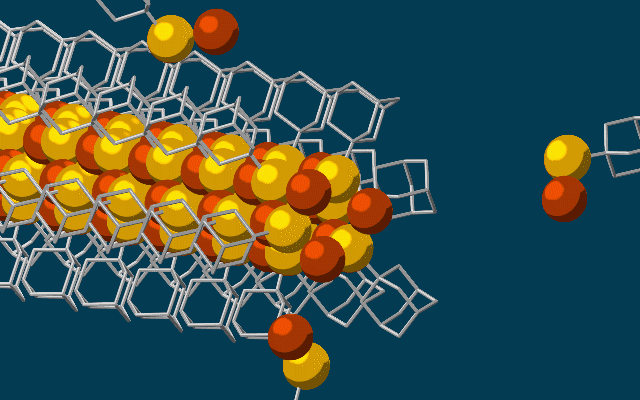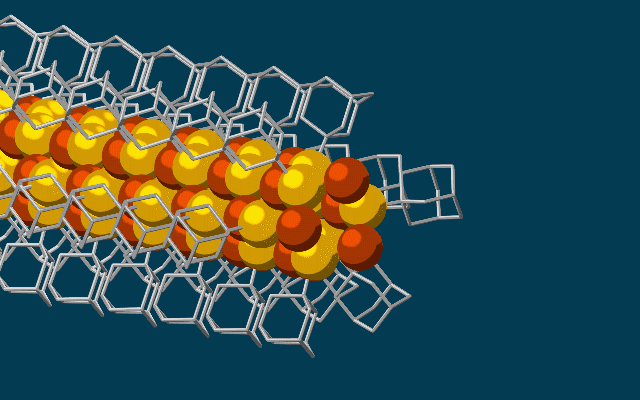
This animation shows molecular building blocks joining the tip of a growing self-assembling nanowire. Each block consists of a diamondoid — the smallest possible bit of diamond — attached to sulfur and copper atoms (yellow and brown spheres). Like LEGO blocks, they only fit together in certain ways that are determined by their size and shape. The copper and sulfur atoms form a conductive wire in the middle, and the diamondoids form an insulating outer shell. (credit: SLAC National Accelerator Laboratory)
Scientists at Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory have discovered a way to use diamondoids* — the smallest possible bits of diamond — to self-assemble atoms, LEGO-style, into the thinnest possible electrical wires, just three atoms wide.
The new technique could potentially be used to build tiny wires for a wide range of applications, including fabrics that generate electricity, optoelectronic devices that employ both electricity and light, and superconducting materials that conduct electricity without any loss. The scientists reported their results last week in Nature Materials.
The researchers started with the smallest possible diamondoids —interlocking cages of carbon and hydrogen — and attached a sulfur atom to each. Floating in a solution, each sulfur atom bonded with a single copper ion — creating a semiconducting combination of copper and sulfur known as a chalcogenide.
A conventional insulated electrical copper wire (credit: Alibaba)
That created the basic nanowire building blocks, which then drifted toward each other, drawn by “unusually strong” van der Waals attraction between the diamondoids, and attached themselves to the growing tip of the nanowire. The attached diamondoids formed an insulating shell — creating the nanoscale equivalent of a conventional insulated electrical wire.
Although there are other ways to get materials to self-assemble, this is the first one shown to make a nanowire with a solid, crystalline core that has good electronic properties, said study co-author Nicholas Melosh, an associate professor at SLAC and Stanford and investigator with SIMES, the Stanford Institute for Materials and Energy Sciences at SLAC.
The team also included researchers from Lawrence Berkeley National Laboratory, the National Autonomous University of Mexico (UNAM) and Justus-Liebig University in Germany. The work was funded by the DOE Office of Science and the German Research Foundation.
* Found naturally in petroleum fluids, they are extracted and separated by size and geometry in a SLAC laboratory.
Citation: Yan et al., Nature Materials, 26 December 2016 (10.1038/nmat4823)
Press Office Contact: Andrew Gordon, [email protected], (650) 926-2282
Abstract of Hybrid metal–organic chalcogenide nanowires with electrically conductive inorganic core through diamondoid-directed assembly
Controlling inorganic structure and dimensionality through structure-directing agents is a versatile approach for new materials synthesis that has been used extensively for metal–organic frameworks and coordination polymers. However, the lack of ‘solid’ inorganic cores requires charge transport through single-atom chains and/or organic groups, limiting their electronic properties. Here, we report that strongly interacting diamondoid structure-directing agents guide the growth of hybrid metal–organic chalcogenide nanowires with solid inorganic cores having three-atom cross-sections, representing the smallest possible nanowires. The strong van der Waals attraction between diamondoids overcomes steric repulsion leading to a cis configuration at the active growth front, enabling face-on addition of precursors for nanowire elongation. These nanowires have band-like electronic properties, low effective carrier masses and three orders-of-magnitude conductivity modulation by hole doping. This discovery highlights a previously unexplored regime of structure-directing agents compared with traditional surfactant, block copolymer or metal–organic framework linkers.