How to rewire the brain with artificial axons to replace damaged pathways
January 21, 2016
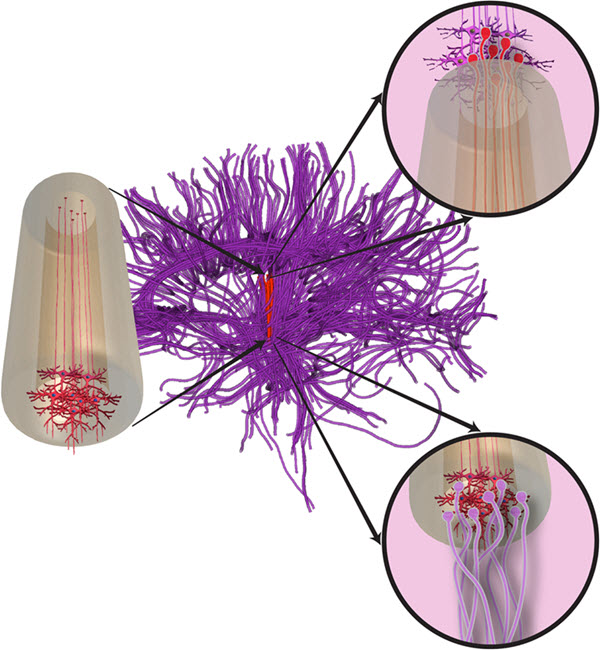
Conceptual schematic of “micro-tissue engineered neural network” (micro-TENN, left) with neurons (red, bottom end) that extend axons (red lines). The new Micro-TENNs (red vertical lines in the middle of the diagram) soften immediately following insertion to better match the mechanical properties of the native brain tissue (purple). The Micro-TENN constructs emulate long axonal pathways with neuronal clusters at the end(s) to allow for integration with native neurons at superficial and deep targets (right, circle insets). (credit: J P Harris et al./Journal of Neural Engineering)
Penn State scientists have grown improved artificial transplantable artificial axons (brain pathways) in the lab. The new “micro-tissue engineered neural networks” (micro-TENNS) replace broken axon pathways when implanted in the brains of rats.
(Neurons are connected by long fibrous projections known as axons. When these connections are damaged, they have very limited capacity to regenerate — unlike many other cells in the body — thus permanently disrupting the body’s signal transmission and communication structure.)
The new lab-grown axons could one day replace damaged axons in the brains of patients with severe head injuries, strokes, or neurodegenerative diseases and could be safely delivered with minimal disruption to brain tissue, according to new research from Penn Medicine’s department of Neurosurgical Research. Their (literally) pathfinding work is published in an open-access paper in the Journal of Neural Engineering.
Senior author D. Kacy Cullen, PhD, an assistant professor of Neurosurgery and his team, previously reported in a 2015 publication in Tissue Engineering that micro-TENNS could be delivered into the brains of rats. The research team has now developed a new, less-invasive delivery method to minimize the body’s reaction and improve the survival and integration of the neural networks.
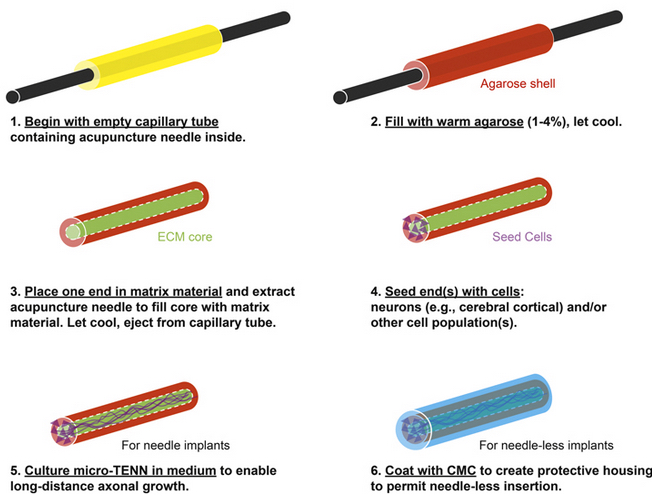
Micro-TENN axon fabrication process (credit: J P Harris et al./Journal of Neural Engineering)
The team replaced needles with a molded cylinder of agarose (a sugar) filled with an extracellular matrix (ECM) supporting structure through which axons — and associated neurons — could grow in the cerebral cortex and the thalamus. The new axons maintained their architecture for several weeks and successfully integrated into existing brain structures, softening immediately following insertion to better match the mechanical properties of the native brain tissue.
Cullen and team plan to perfect their processes and further integrate neuroscience and engineering to come up with unique ways to aid patients suffering from brain injury or common neurodegenerative diseases. “Additional research is required to directly test micro-TENN neuron survival and integration for each of these insertion methods,” Cullen said.
“We hope this regenerative medicine strategy will someday enable us to grow individualized neural networks that are tailored for each patient’s specific need,” he said, “ultimately, to replace lost neural circuits and improve brain function.”
Abstract of Advanced biomaterial strategies to transplant preformed micro-tissue engineered neural networks into the brain
Objective. Connectome disruption is a hallmark of many neurological diseases and trauma with no current strategies to restore lost long-distance axonal pathways in the brain. We are creating transplantable micro-tissue engineered neural networks (micro-TENNs), which are preformed constructs consisting of embedded neurons and long axonal tracts to integrate with the nervous system to physically reconstitute lost axonal pathways. Approach. We advanced micro-tissue engineering techniques to generate micro-TENNs consisting of discrete populations of mature primary cerebral cortical neurons spanned by long axonal fascicles encased in miniature hydrogel micro-columns. Further, we improved the biomaterial encasement scheme by adding a thin layer of low viscosity carboxymethylcellulose (CMC) to enable needle-less insertion and rapid softening for mechanical similarity with brain tissue. Main results. The engineered architecture of cortical micro-TENNs facilitated robust neuronal viability and axonal cytoarchitecture to at least 22 days in vitro. Micro-TENNs displayed discrete neuronal populations spanned by long axonal fasciculation throughout the core, thus mimicking the general systems-level anatomy of gray matter—white matter in the brain. Additionally, micro-columns with thin CMC-coating upon mild dehydration were able to withstand a force of 893 ± 457 mN before buckling, whereas a solid agarose cylinder of similar dimensions was predicted to withstand less than 150 μN of force. This thin CMC coating increased the stiffness by three orders of magnitude, enabling needle-less insertion into brain while significantly reducing the footprint of previous needle-based delivery methods to minimize insertion trauma. Significance. Our novel micro-TENNs are the first strategy designed for minimally invasive implantation to facilitate nervous system repair by simultaneously providing neuronal replacement and physical reconstruction of long-distance axon pathways in the brain. The micro-TENN approach may offer the ability to treat several disorders that disrupt the connectome, including Parkinson’s disease, traumatic brain injury, stroke, and brain tumor excision.
Abstract of Rebuilding Brain Circuitry with Living Micro-Tissue Engineered Neural Networks
Prominent neuropathology following trauma, stroke, and various neurodegenerative diseases includes neuronal degeneration as well as loss of long-distance axonal connections. While cell replacement and axonal pathfinding strategies are often explored independently, there is no strategy capable of simultaneously replacing lost neurons and re-establishing long-distance axonal connections in the central nervous system. Accordingly, we have created micro-tissue engineered neural networks (micro-TENNs), which are preformed constructs consisting of long integrated axonal tracts spanning discrete neuronal populations. These living micro-TENNs reconstitute the architecture of long-distance axonal tracts, and thus may serve as an effective substrate for targeted neurosurgical reconstruction of damaged pathways in the brain. Cerebral cortical neurons or dorsal root ganglia neurons were precisely delivered into the tubular constructs, and properties of the hydrogel exterior and extracellular matrix internal column (180–500 μm diameter) were optimized for robust neuronal survival and to promote axonal extensions across the 2.0 cm tube length. The very small diameter permits minimally invasive delivery into the brain. In this study, preformed micro-TENNs were stereotaxically injected into naive rats to bridge deep thalamic structures with the cerebral cortex to assess construct survival and integration. We found that micro-TENN neurons survived at least 1 month and maintained their long axonal architecture along the cortical–thalamic axis. Notably, we also found neurite penetration from micro-TENN neurons into the host cortex, with evidence of synapse formation. These micro-TENNs represent a new strategy to facilitate nervous system repair by recapitulating features of neural pathways to restore or modulate damaged brain circuitry.