How to use graphene as a biosensor by increasing its chemical selectivity
June 25, 2015
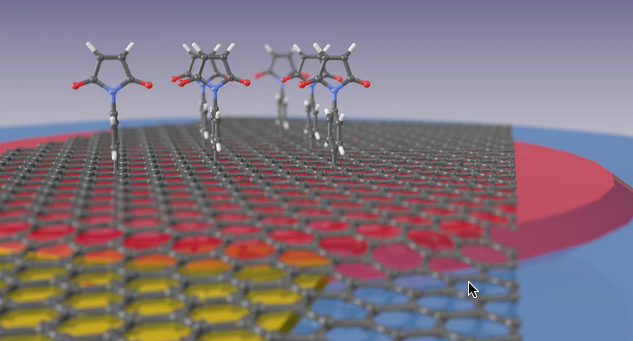
The illustration shows how maleimide compounds (top) bind to the graphene surface to hold detector molecules. The graphene monolayer lies on a thin film of silicon nitride (red) that in turn is on a quartz microbalance (blue) and the graphene can be subjected to voltage via a gold contact (yellow). (credit: Marc Gluba/HZB)
Scientists at the HZB Institute for Silicon Photovoltaics in Berlin have succeeded in precisely measuring and controlling the thickness of an organic compound that has been bound to a graphene layer. This could enable graphene to be used as a sensitive detector for biological molecules in the future.
It has long been known that graphene is useful for detecting traces of organic molecules, because the electrical conductivity of graphene drops as soon as foreign molecules bind to it. The problem: graphene is not very selective, making it difficult to differentiate molecules.
The scientists found a way to increase the selectivity by electrochemically connecting graphene to host molecules that act as detector molecules functioning as selective binding sites. To accomplish this, para-maleimidophenyl groups (maleimide) from an organic solution were grafted to the surface of the graphene. These organic molecules behave like mounting brackets to which the selective detector molecules can be attached in the next step.
“Thanks to these molecules, graphene can now be employed for detecting various substances, similar to how a key fits a lock,” explains researcher Marc Gluba. The “lock” molecules on the surface are highly selective and absorb only the matching “key” molecules, allowing for accurately measuring how many molecules actually were grafted to the surface of the graphene.
One use would be an inexpensive “lab-on-a-chip.” Using a single drop of blood could immediately provide data for medical diagnosis, says Prof. Norbert Nickel, head of the research team.
Abstract of Quantifying the electrochemical maleimidation of large area graphene
The covalent modification of large-area graphene sheets by p-(N-Maleimido)phenyl (p-MP) via electrochemical grafting of p-(N-Maleimido)benzenediazonium tetrafluoroborate (p-MBDT) is successfully demonstrated for the first time. The deposition process is monitored in-situ using the mass change of a graphene/SiNX:H/Au-coated quartz crystal microbalance(QCM) chip. The resulting mass increase correlates with a maleimide thickness of approximately 2.3 molecular layers. The presence of an infrared absorption band at 1726 cm-1 shows that maleimide groups were deposited on the substrates. Raman backscattering spectra reveal the presence of D and D′ modes of the graphene layer, indicating that p-MP forms covalent bonds to graphene. Using the mass change and charge transfer during the potential cycling the faradaic efficiency of the functionalisation process was deduced, which amounts to eta = 22%.