Implantable brain electronics is here
June 10, 2015
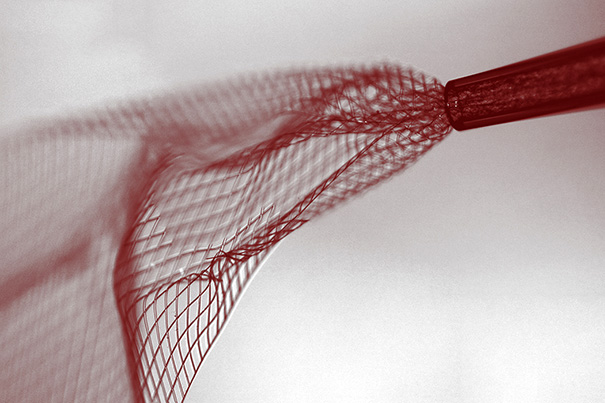
Mesh electronics being injected through sub-100 micrometer inner diameter glass needle into aqueous solution (credit: Lieber Research Group, Harvard University)
In a world first, U.S. and Chinese scientists have developed a method to inject microelectronic devices such as wires and transistors directly into the brain (or other body parts) to measure or stimulate neural activity. The new method could lead to sophisticated new ways to treat conditions ranging from neurodegenerative disorders to paralysis.
Developed by researchers in Charles Lieber’s lab at Harvard University and the National Center for Nanoscience and Technology in Beijing, the invention is based on a simple but radical concept: injecting a biocompatible polymer scaffold mesh with attached microelectronic devices into the brain via syringe.
How to inject electronics into the brain
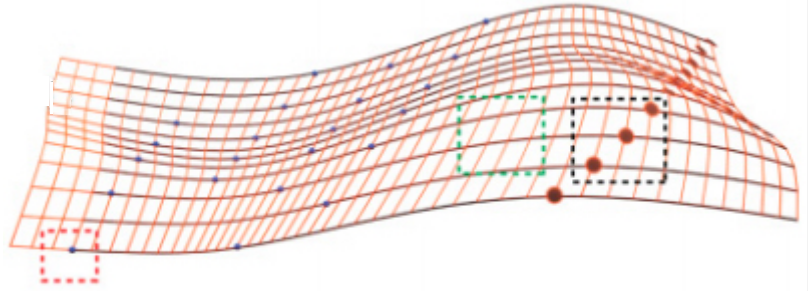
Schematic of an injectable mesh electronics structure. The red-orange lines highlight the overall mesh structure. The (roughly) horizontal black lines indicate metal interconnects between input/output (I/O) pads (black filled circles) and recording devices (blue filled circles). The red dashed box highlights two different types of devices — electrochemical and field-effect transistors (FETs), the green dashed box highlights mesh network metal interconnects, and the black dashed box highlights input-output (connecting) pads. (credit: Jia Liu et al./Nature Nanotechnology)
The process for fabricating the scaffold is similar to that used to etch microchips, and begins with a dissolvable layer deposited on a biocompatible nanoscale polymer mesh substrate, with embedded nanowires, transistors, and other microelectronic devices attached. The mesh is then tightly rolled up, allowing it to be sucked up into a syringe via a thin glass needle. The mesh can then be injected into brain (or other) tissue by the syringe.
The input-output connection of the mesh electronics can be connected to standard electronics devices (for voltage insertion or measurement, for example), allowing the mesh-embedded devices to be individually addressed and used to precisely stimulate or record individual neural activity.
Electrodes that can be inserted in the brain are already in use, such as neural stimulators for Parkinson’s disease, for example, but they are “dumb” devices that must be connected via a wire to external electronic devices to do anything. Also, these existing techniques are “crude relative to the way the brain is wired,” said research study leader Charles Lieber, PhD, the Harvard Mark Hyman Jr. Professor of Chemistry,. “Whether it’s a silicon probe or flexible polymers … they cause inflammation in the tissue that requires periodically changing the position or the stimulation.
“Neuro-philic” electronics
“But with our injectable electronics, it’s as if it’s not there at all. They are one million times more flexible than any state-of-the-art flexible electronics and have subcellular feature sizes. They’re what I call ‘neuro-philic’ — they actually like to interact with neurons.”
The researchers tested the concept with mice.
“I do feel that this has the potential to be revolutionary,” Lieber said. “This opens up a completely new frontier where we can explore the interface between electronic structures and biology. For the past 30 years, people have made incremental improvements in micro-fabrication techniques that have allowed us to make rigid probes smaller and smaller, but no one has addressed this issue — the electronics/cellular interface — at the level at which biology works.”
In an earlier study, scientists in Lieber’s lab demonstrated that cardiac or nerve cells grown with embedded scaffolds could be used to create “cyborg” tissue. Researchers were then able to record electrical signals generated by the tissue, and to measure changes in those signals as they administered cardio- or neuro-stimulating drugs.
“We were able to demonstrate that we could make this scaffold and culture cells within it, but we didn’t really have an idea how to insert that into pre-existing tissue,” Lieber said. “But if you want to study the brain or develop the tools to explore the brain-machine interface, you need to stick something into the body. When releasing the electronic scaffold completely from the fabrication substrate, we noticed that it was almost invisible and very flexible, like a polymer, and could literally be sucked into a glass needle or pipette. From there, we simply asked, ‘Would it be possible to deliver the mesh electronics by syringe needle injection?’”
“These type of things have never been done before, from both a fundamental neuroscience and medical perspective,” Lieber said. “It’s really exciting — there are a lot of potential applications.”
Going forward, researchers hope to better understand how the body reacts to the injectable electronics over longer periods. “The idea of being able to precisely position and record from very specific areas, or even from specific neurons over an extended period of time — this could, I think, make a huge impact on neuroscience,” Lieber said.
The research is described in a June 8 paper in Nature Nanotechnology. Harvard’s Office of Technology Development has filed for a provisional patent on the technology and is actively seeking commercialization opportunities.
Abstract of Syringe-injectable electronics
Seamless and minimally invasive three-dimensional interpenetration of electronics within artificial or natural structures could allow for continuous monitoring and manipulation of their properties. Flexible electronics provide a means for conforming electronics to non-planar surfaces, yet targeted delivery of flexible electronics to internal regions remains difficult. Here, we overcome this challenge by demonstrating the syringe injection (and subsequent unfolding) of sub-micrometre-thick, centimetre-scale macroporous mesh electronics through needles with a diameter as small as 100 μm. Our results show that electronic components can be injected into man-made and biological cavities, as well as dense gels and tissue, with >90% device yield. We demonstrate several applications of syringe-injectable electronics as a general approach for interpenetrating flexible electronics with three-dimensional structures, including (1) monitoring internal mechanical strains in polymer cavities, (2) tight integration and low chronic immunoreactivity with several distinct regions of the brain, and (3) in vivo multiplexed neural recording. Moreover, syringe injection enables the delivery of flexible electronics through a rigid shell, the delivery of large-volume flexible electronics that can fill internal cavities, and co-injection of electronics with other materials into host structures, opening up unique applications for flexible electronics.