Implanted neuronal stem cells generate neurons and synapses, becoming a functioning part of mouse brain
August 13, 2014
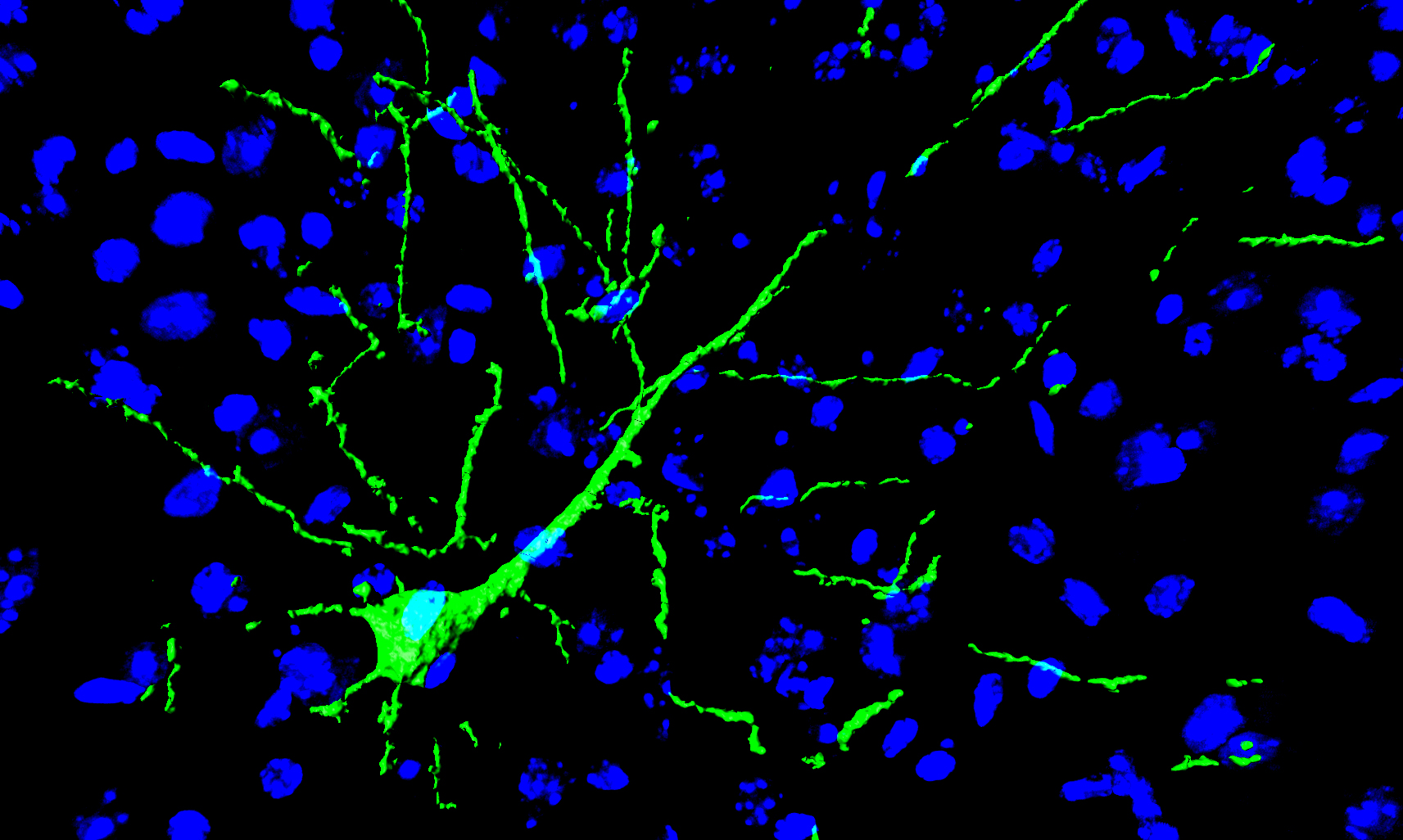
Part of a brain slice showing a functioning transplanted induced neural stem cell (green) fully integrated in the neuronal network of the brain (blue) (credit: LCSB)
Scientists at the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg have grafted induced neuronal stem cells (iNSC) into the brains of mice, with long-term functionality and stability, for the first time. Six months after implantation, the new neurons, reprogrammed from skin cells, became fully and functionally integrated into the brain, creating synapses and glial cells.
This successful implantation of neurons raises hope for future therapies for neurodegenerative diseases, replacing sick neurons with healthy ones — in the brains of Parkinson’s disease patients, for example. However, “successes in human therapy are still a long way off,” cautioned principal investigator Prof. Jens Schwamborn.
The researchers published their results in Stem Cell Reports (open access).
Fully integrated into the brain, connected by synapses
The treated mice showed no adverse side effects, even six months after implantation into the hippocampus and cortex regions of the brain, according to the researchers. The neurons exhibited normal activity and were fully integrated into the complex network of the brain, connected to the original brain cells via newly formed synapses.
In addition, the iNSCs are not predisposed to tumor formation, as in the case of induced pluripotent stem cells (iPSCs).
“Building upon the current insights, we will now be looking specifically at the type of neurons that die off in the brain of Parkinson’s patients — namely, the dopamine-producing neurons,” Schwamborn said. He said that in the future, implanted neurons could produce dopamine (which is lacking in Parkinson’s) directly in the patient’s brain and transport it to the appropriate sites — constituting an actual cure.
Colleagues from the Max Planck Institute, University Hospital Münster, and the University of Bielefeld were also involved in the research.
Abstract of Stem Cell Reports paper
- In vivo long-term survival of transplanted induced neural stem cells
- Lack of tumorigenic outgrowth
- In vivo multilineage differentiation of transplanted iNSCs
- Functional integration, synapse formation, and electrophysiological activity
Differentiated cells can be converted directly into multipotent neural stem cells (i.e., induced neural stem cells [iNSCs]). iNSCs offer an attractive alternative to induced pluripotent stem cell (iPSC) technology with regard to regenerative therapies. Here, we show an in vivo long-term analysis of transplanted iNSCs in the adult mouse brain. iNSCs showed sound in vivo long-term survival rates without graft overgrowths. The cells displayed a neural multilineage potential with a clear bias toward astrocytes and a permanent downregulation of progenitor and cell-cycle markers, indicating that iNSCs are not predisposed to tumor formation. Furthermore, the formation of synaptic connections as well as neuronal and glial electrophysiological properties demonstrated that differentiated iNSCs migrated, functionally integrated, and interacted with the existing neuronal circuitry. We conclude that iNSC long-term transplantation is a safe procedure; moreover, it might represent an interesting tool for future personalized regenerative applications.