Injected into the body, self-healing nanogel acts as customized long-term drug supply
February 23, 2015
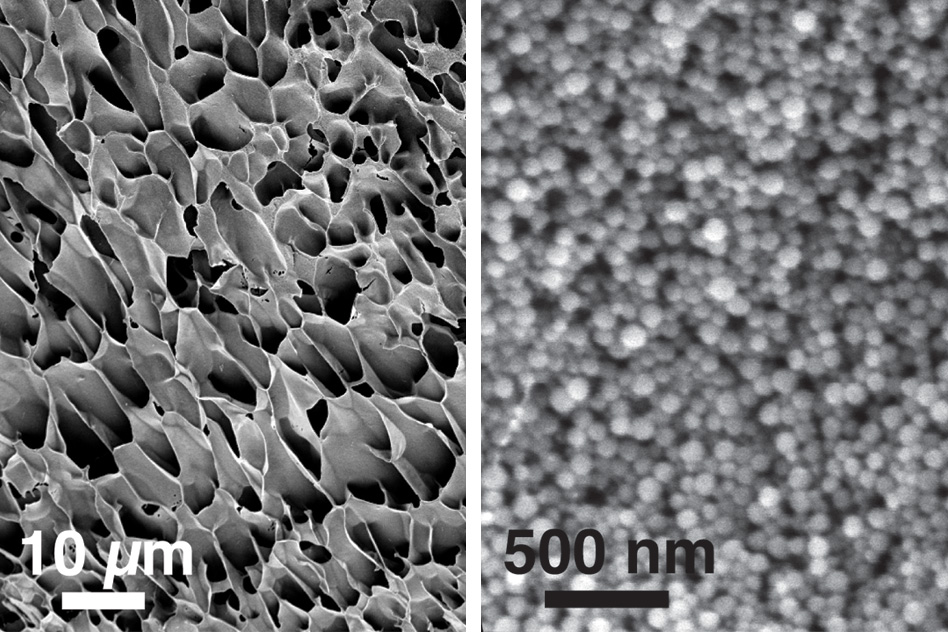
These scanning electron microscopy images, taken at different magnifications, show the structure of new hydrogels made of nanoparticles interacting with long polymer chains (credit: Eric A. Appel et al./Nature Communications)
MIT chemical engineers have designed a new type of self-healing hydrogel that can be injected through a syringe to supply one or two different drugs at a time.
In theory, gels could be useful for delivering drugs for treating cancer, macular degeneration, or heart disease because they can be molded into specific shapes and designed to release their payload in a specific location over a specified time period. However, current gels are not very practical because they must be implanted surgically.
In contrast, the new gel consists of a mesh network of nanoparticles made of polymers entwined within strands of another polymer, such as cellulose. “Now you have a gel that can change shape when you apply stress to it, and then, importantly, it can re-heal when you relax those forces. That allows you to squeeze it through a syringe or a needle and get it into the body without surgery,” says Mark Tibbitt, a postdoc at MIT’s Koch Institute for Integrative Cancer Research and one of the lead authors of a paper describing the gel in Nature Communications on Thursday Feb. 19.
Koch Institute postdoc Eric Appel is also a lead author of the paper, and the paper’s senior author is Robert Langer, the David H. Koch Institute Professor at MIT.
Another limitation of hydrogels for biomedical uses — such as making soft contact lenses — is that they are traditionally formed by irreversible chemical linkages between polymers, so their shape cannot easily be altered.
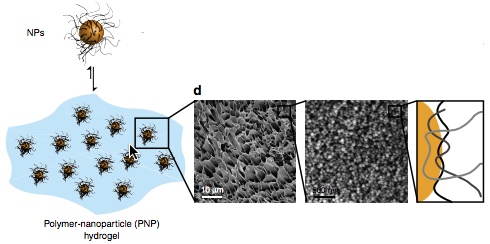
Cryogenic scanning electron microscopy images (progressively magnified) reveal that polymer-nanoparticles (PNP) self-assemble into a hydrogel (blue), forming a network held together by multivalent, dynamic polymer-nanoparticle interactions (polymer chains: gray; nanoparticles: orange). (credit: Eric A. Appel et al./Nature Communications)
So the MIT team set out to create a gel that could survive strong mechanical forces, known as shear forces, yet capable of reforming itself. Other researchers have created such gels by engineering proteins that self-assemble into hydrogels, but this approach requires complex biochemical processes. The MIT team wanted to design something simpler.
The MIT approach relies on a combination of two readily available components. One is a type of nanoparticle formed of PEG-PLA copolymers, first developed in Langer’s lab decades ago and now commonly used to package and deliver drugs. To form the new hydrogel, the researchers mixed these particles with a polymer — in this case, cellulose.
Each polymer chain forms weak bonds with many nanoparticles, producing a loosely woven lattice or network of polymers and nanoparticles. Because each attachment point is fairly weak, the bonds are able to break apart under mechanical stress, such as when injected through a syringe. When these shear forces are over, the polymers and nanoparticles reassemble, forming new attachments with different partners and healing the gel.
Using two components to form the gel also gives the researchers the opportunity to deliver two different drugs at the same time. PEG-PLA nanoparticles have an inner core that is ideally suited to carry hydrophobic (water-incompatible) small-molecule drugs, which include many chemotherapy drugs. Meanwhile, the polymers, which exist in a watery solution, can carry hydrophilic (water-compatible) molecules such as proteins, including antibodies and growth factors.
Long-term drug delivery
In this study, the researchers showed that the gels survived injection under the skin of mice and successfully released two drugs, one hydrophobic and one hydrophilic, over several days.
This type of gel offers an important advantage over injecting a liquid solution of drug-delivery nanoparticles: Such a solution will immediately disperse throughout the body, while the gel stays in place after injection, allowing the drug to be targeted to a specific tissue and avoiding toxic reactions elsewhere. Furthermore, the properties of each gel component can be tuned so the drugs they carry are released at different rates, allowing them to be tailored for different uses.
Treating eye, heart, and cancer issues
The researchers are now looking into using the gel to deliver anti-angiogenesis (anti-blood-vessel-forming) drugs to treat macular degeneration. Currently, patients receive these drugs, which cut off the growth of blood vessels that interfere with sight, as an injection into the eye once a month (try not to visualize that). The MIT team envisions that the new gel could be programmed to deliver these drugs over several months, reducing the frequency of injections.
Another potential application for the gels is delivering drugs, such as growth factors, that could help repair damaged heart tissue after a heart attack.
The researchers are also pursuing the possibility of using this gel to deliver cancer drugs to kill tumor cells that get left behind after surgery. In that case, the gel would be loaded with a chemical that lures cancer cells toward the gel, as well as a chemotherapy drug that would kill them. This could help eliminate the residual cancer cells that often form new tumors following surgery.
“Removing the tumor leaves behind a cavity that you could fill with our material, which would provide some therapeutic benefit over the long term in recruiting and killing those cells,” Appel says. “We can tailor the materials to provide us with the drug-release profile that makes it the most effective at actually recruiting the cells.”
The research was funded by the Wellcome Trust, the Misrock Foundation, the Department of Defense, and the National Institutes of Health.
Abstract of Self-assembled hydrogels utilizing polymer–nanoparticle interactions
Mouldable hydrogels that flow on applied stress and rapidly self-heal are increasingly utilized as they afford minimally invasive delivery and conformal application. Here we report a new paradigm for the fabrication of self-assembled hydrogels with shear-thinning and self-healing properties employing rationally engineered polymer–nanoparticle (NP) interactions. Biopolymer derivatives are linked together by selective adsorption to NPs. The transient and reversible interactions between biopolymers and NPs enable flow under applied shear stress, followed by rapid self-healing when the stress is relaxed. We develop a physical description of polymer–NP gel formation that is utilized to design biocompatible gels for drug delivery. Owing to the hierarchical structure of the gel, both hydrophilic and hydrophobic drugs can be entrapped and delivered with differential release profiles, both in vitro and in vivo. The work introduces a facile and generalizable class of mouldable hydrogels amenable to a range of biomedical and industrial applications.