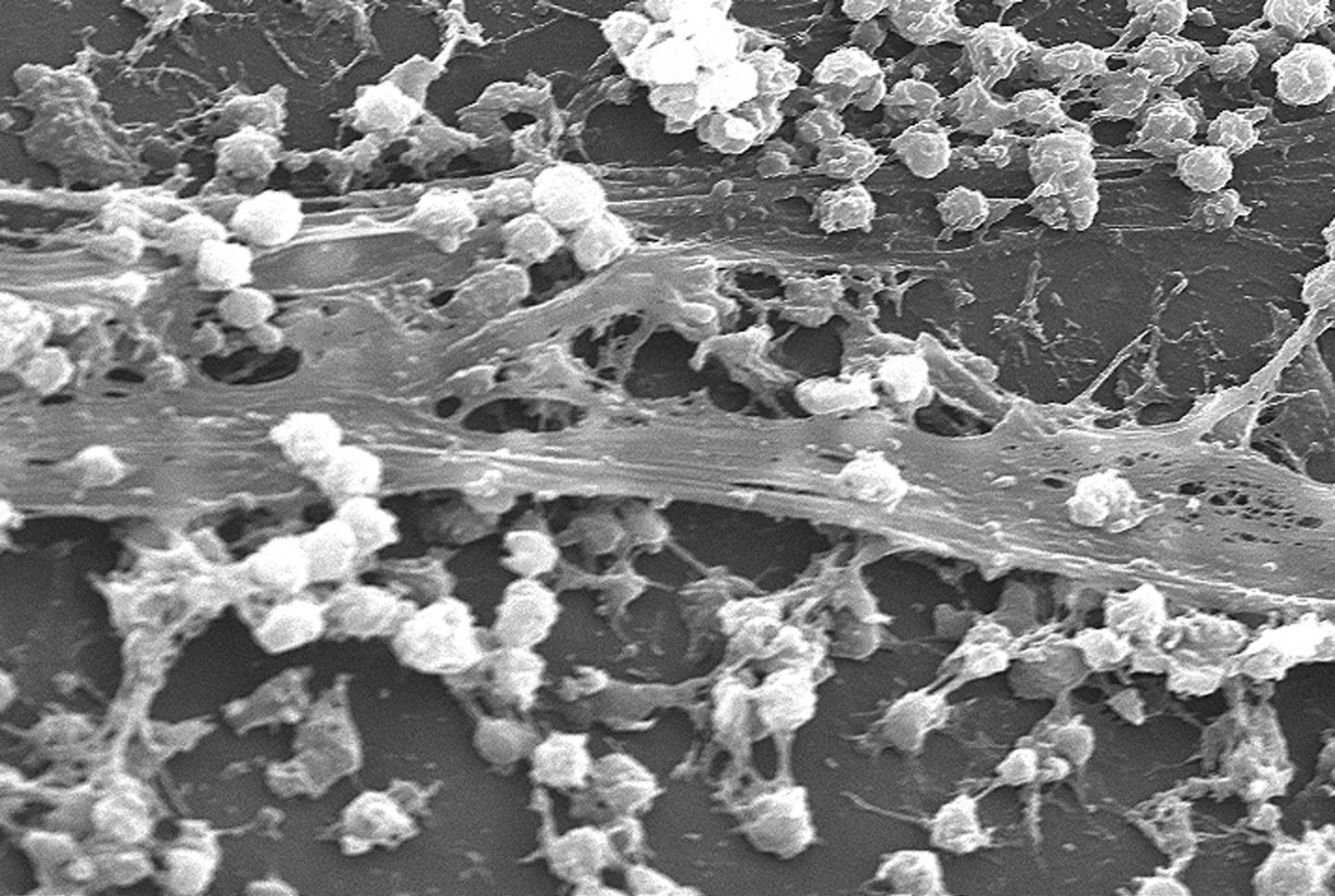
Magnetic nanoparticles combat biofilms, a source of chronic bacterial infections
December 21, 2015
A solution for biofilms* — a scourge of infections in hospitals and kitchens formed by bacteria that stick to each other on living tissue and medical instruments — has been developed by University of New South Wales researchers: Injecting iron oxide nanoparticles into the biofilms, and using an applied magnetic field to heat them, triggering them into dispersing.
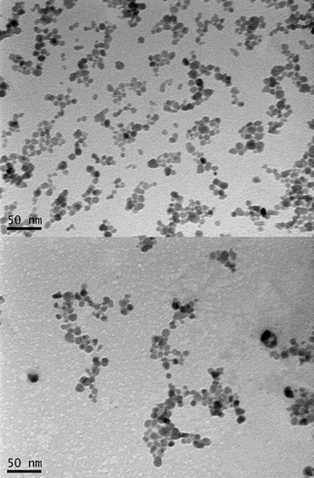
Transmission electron microscopy (TEM) micrographs of dried nanoparticles before (top) and after (bottom) conjugation with polymer (credit: Thuy-Khanh Nguyen et al./Scientific Reports)
“Chronic biofilm-based infections are often extremely resistant to antibiotics and many other conventional antimicrobial agents, and have a high capacity to evade the body’s immune system,” said Associate Professor Cyrille Boyer of the School of Chemical Engineering and deputy director of Australian Centre for NanoMedicine. “Our study points to a pathway for the non-toxic dispersal of biofilms in infected tissue, while also greatly improving the effect of antibiotic therapies.”
When biofilms want to colonize a new site, they disperse into individual cells, reducing the protective action of the biofilm. It is this process the UNSW team sought to trigger. They achieved this using iron oxide nanoparticles coated with polymers that help stabilize and maintain the nanoparticles in a dispersed state, making them an ideal non-toxic tool for treating biofilm infection.
Once dispersed, the bacteria are easier to deal with, creating the potential to remove recalcitrant, antimicrobial-tolerant biofilm infections with antimicrobial agents.
The discovery of how to dislodge biofilms by the UNSW Faculty of Engineering team was made using the opportunistic human pathogen Pseudomonas aeruginosa. This is a model organism whose response to the technique the researchers believe will apply to most other bacteria.
“The use of these polymer-coated iron oxide nanoparticles to disperse biofilms may have broad applications across a range of clinical and industrial settings,” said Boyer.
The research appears in an open-access paper today (Dec. 21) in Nature’s Scientific Reports.
* Biofilms have been linked to 80% of infections, forming on living tissues (e.g. respiratory, gastrointestinal and urinary tracts, oral cavities, eyes, ears, wounds, heart and cervix) or dwelling in medical devices (e.g. dialysis catheters, prosthetic implants and contact lenses).
The formation of biofilms is a growing and costly problem in hospitals, creating infections that are more difficult to treat — leading to chronic inflammation, impaired wound healing, rapidly acquired antibiotic resistance and the spread of infectious embolisms in the bloodstream.
They also cause fouling and corrosion of wet surfaces, and the clogging of filtration membranes in sensitive equipment, even posing a threat to public health by acting as reservoirs of pathogens in distribution systems for drinking water.
In general, bacteria have two life forms during growth and proliferation: planktonic, where bacteria exist as single, independent cells; or aggregated together in colonies as biofilms, where bacteria grow in a slime-like polymer matrix that protects them from the environment around them.
Acute infections mostly involve planktonic bacteria, which are usually treatable with antibiotics. However, when bacteria have had enough time to form a biofilm — within a human host or non-living material such as dialysis catheters – an infection can often become untreatable and develop into a chronic state.
UNSW | 2015 Malcolm McIntosh Prize for Physical Scientist of the Year
Abstract of Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy
The dispersal phase that completes the biofilm lifecycle is of particular interest for its potential to remove recalcitrant, antimicrobial tolerant biofilm infections. Here we found that temperature is a cue for biofilm dispersal and a rise by 5 °C or more can induce the detachment of Pseudomonas aeruginosa biofilms. Temperature upshifts were found to decrease biofilm biomass and increase the number of viable freely suspended cells. The dispersal response appeared to involve the secondary messenger cyclic di-GMP, which is central to a genetic network governing motile to sessile transitions in bacteria. Furthermore, we used poly((oligo(ethylene glycol) methyl ether acrylate)-block-poly(monoacryloxy ethyl phosphate)-stabilized iron oxide nanoparticles (POEGA-b-PMAEP@IONPs) to induce local hyperthermia in established biofilms upon exposure to a magnetic field. POEGA-b-PMAEP@IONPs were non-toxic to bacteria and when heated induced the detachment of biofilm cells. Finally, combined treatments of POEGA-b-PMAEP@IONPs and the antibiotic gentamicin reduced by 2-log the number of colony-forming units in both biofilm and planktonic phases after 20 min, which represent a 3.2- and 4.1-fold increase in the efficacy against planktonic and biofilm cells, respectively, compared to gentamicin alone. The use of iron oxide nanoparticles to disperse biofilms may find broad applications across a range of clinical and industrial settings.