Major steps toward a bioengineered heart for transplantation
March 17, 2016
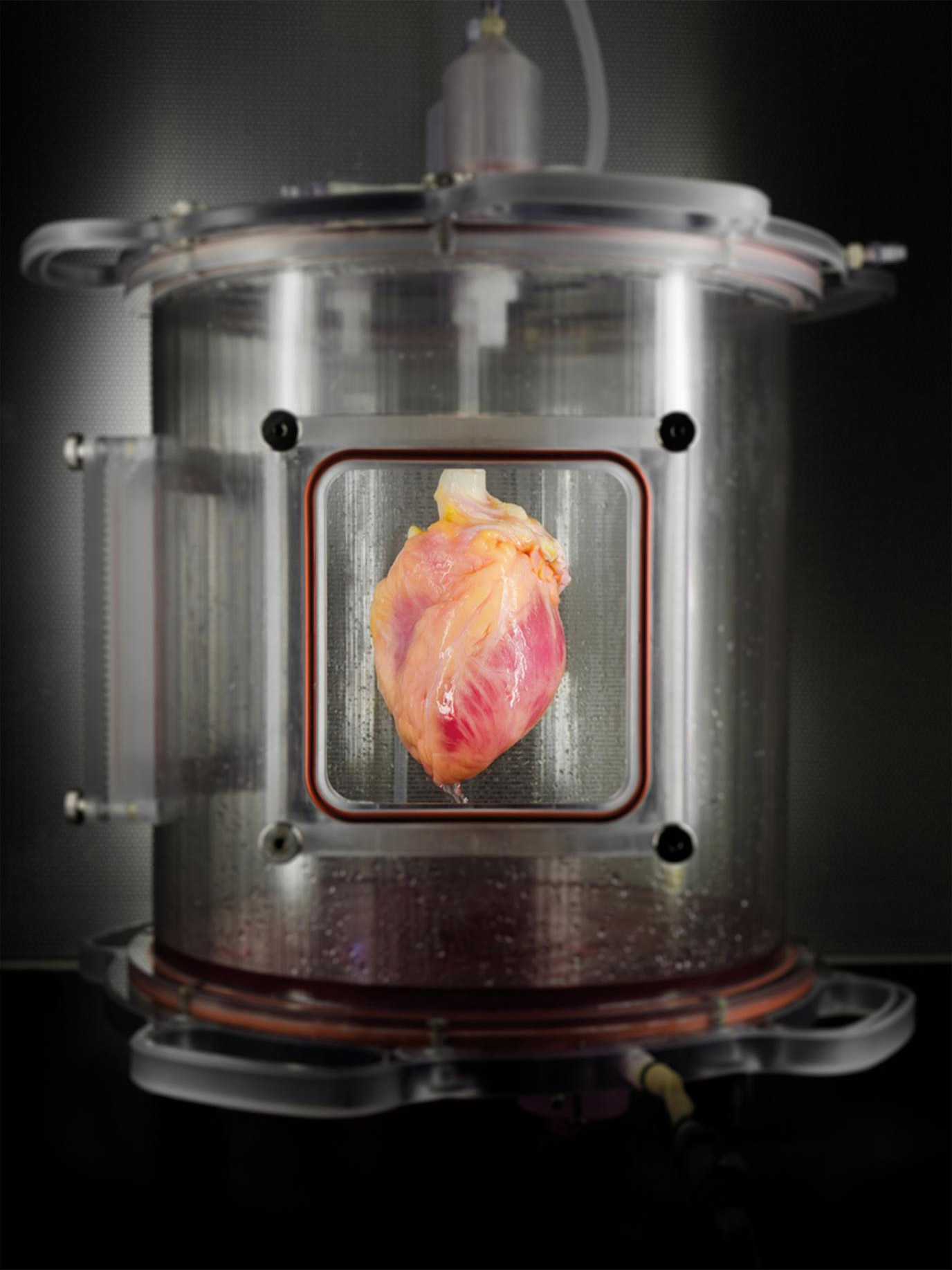
A human whole heart was stripped of cells, leaving behind a “structural scaffold” of connective tissue, which was then partially re-seeded with human heart-like cells grown in the laboratory from skin cells. Once these new heart-like cells were re-seeded into the heart scaffold, the heart was regenerated in this bioreactor, which delivers a nutrient solution and replicates some of the environmental conditions in a living heart. (credit: Bernhard Jank, MD/Ott Lab, Center for Regenerative Medicine, Massachusetts General Hospital)
Massachusetts General Hospital (MGH) researchers have taken early steps towards producing a bioengineered heart for transplantation that would use cells from the patient receiving the heart.
Using a patient’s own cells would help to overcome some of the problems associated with receiving a heart donated by another person, including immune rejection of the donated heart, as well as the long-term side effects of life-long treatment with the immunosuppressive drugs needed to suppress the immune system and reduce the risk of rejection.
To achieve those steps, Jacques Guyette, PhD, of the Massachusetts General Hospital’s Center for Regenerative Medicine, lead author of a paper in Circulation Research, says the research team had to overcome three major technical challenges.
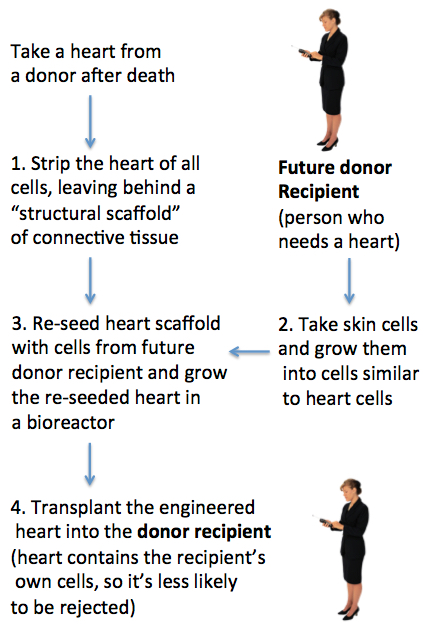
Future methodology for producing bioengineered hearts (credit: Julie Milland)
1. Create a structural scaffold from a human heart
One challenge is producing a structural scaffold able to support new functioning heart cells. To do this, the researchers take a human heart and remove the heart muscle and other cells and components that would stimulate the recipient’s immune system to reject the organ. Once stripped, the structural and connective tissues that give the heart its 3D structure are left behind.
If researchers can re-seed this structural scaffold with viable heart-like cells from the patient who will receive the heart (steps 2 and 3 below), the engineered heart would have the potential to reduce the risk of rejection and the resulting need for long-term immunosuppressive treatment.
Team leader Harald Ott, MD, an assistant professor of surgery at Harvard Medical School, has pioneered a method for stripping the living cells from donor organs and then re-populating the remaining scaffold with new cells.*
2. Grow cells that will function and contract like heart cells
Another challenge is developing a method that will enable researchers to use cells from the potential heart recipient to produce cells that will function like heart cells. These are generated from induced pluripotent stem cells (iPSCs) — cells with the potential to be turned into many different types of cells.
The team generated the heart-like muscle cells from reprogrammed skin cells. Once they had checked the quality of these cells, they grew them in the laboratory for several days and showed that the cells developed into tissue that spontaneously contracted like heart cells.**
3. Re-seed the human heart scaffold and grow it in an automated bioreactor
The final challenge was developing an automated bioreactor system capable of supporting a whole human heart while the re-seeded heart cells take hold.*** In this initial study, the researchers only partially re-seeded the scaffold — many more cells would be needed to totally re-populate a functioning heart scaffold.
After incubating the engineered heart in the bioreactor, the researchers showed that the regenerated tissue behaved like immature cardiac muscle tissue that was able to contract in response to electrical stimulation.
Next steps
“Regenerating a whole heart is most certainly a long-term goal that is several years away,” says Guyette. “Among the next steps that we are pursuing are improving methods to generate even more cardiac cells.”
He says re-seeding a whole heart would take tens of billions of cells, so the team needs to optimize the bioreactor techniques to improve the maturation and function of engineered cardiac tissue. They also need to integrate the electrical function of the regenerated tissue in the bioengineered heart. In the meantime, Guyette says the team is working on engineering a functional myocardial patch that could be used to replace tissue damaged after a heart attack or heart failure.
The study was supported by National Institutes of Health Director’s New Innovator Award and by National Heart Lung and Blood Institute grants.
* Since 2008, Ott’s team has used the approach to generate functional rat kidneys and lungs and has stripped cells from large-animal hearts, lungs and kidneys.
This study used 73 human hearts donated through the New England Organ Bank, unsuitable for transplantation and recovered under research consent. Using a scaled-up version of a process originally developed in rat hearts, the team stripped cells from the hearts from both brain-dead donors and from those who had undergone cardiac death. The consequent cardiac scaffolds showed a high retention of matrix proteins and preserved blood vessels. The structure was free of cardiac cells and other molecules that could induce rejection.
** Instead of using genetic manipulation to generate iPSCs from adult cells, the team used a newer method to reprogram skin cells, which should be both more efficient and less likely to run into regulatory hurdles. They then induced the iPSCs to create cardiac muscle cells or cardiomyocytes, documenting patterns of gene expression that reflected developmental milestones and generating cells in sufficient quantity for possible clinical application. Cardiomyocytes were then reseeded into three-dimensional matrix tissue, first into thin matrix slices and then into 15 mm fibers, which developed into spontaneously contracting tissue after several days in culture.
*** The team delivered about 500 million heart-like cells grown from iPSC into the left ventricular wall of the heart scaffolds. The re-seeded organs were mounted for 14 days in an automated bioreactor system developed by the team. The automated bioreactor system supplied the organ with a circulating nutrient solution and applied environmental conditions to reproduce conditions within a living heart.
Abstract of Bioengineering Human Myocardium on Native Extracellular Matrix
Rationale: More than 25 million individuals have heart failure worldwide, with ≈4000 patients currently awaiting heart transplantation in the United States. Donor organ shortage and allograft rejection remain major limitations with only ≈2500 hearts transplanted each year. As a theoretical alternative to allotransplantation, patient-derived bioartificial myocardium could provide functional support and ultimately impact the treatment of heart failure.
Objective: The objective of this study is to translate previous work to human scale and clinically relevant cells for the bioengineering of functional myocardial tissue based on the combination of human cardiac matrix and human induced pluripotent stem cell–derived cardiomyocytes.
Methods and Results: To provide a clinically relevant tissue scaffold, we translated perfusion-decellularization to human scale and obtained biocompatible human acellular cardiac scaffolds with preserved extracellular matrix composition, architecture, and perfusable coronary vasculature. We then repopulated this native human cardiac matrix with cardiomyocytes derived from nontransgenic human induced pluripotent stem cells and generated tissues of increasing 3-dimensional complexity. We maintained such cardiac tissue constructs in culture for 120 days to demonstrate definitive sarcomeric structure, cell and matrix deformation, contractile force, and electrical conduction. To show that functional myocardial tissue of human scale can be built on this platform, we then partially recellularized human whole-heart scaffolds with human induced pluripotent stem cell–derived cardiomyocytes. Under biomimetic culture, the seeded constructs developed force-generating human myocardial tissue and showed electrical conductivity, left ventricular pressure development, and metabolic function.