Mapping connections of single neurons using a holographic light beam
November 13, 2017
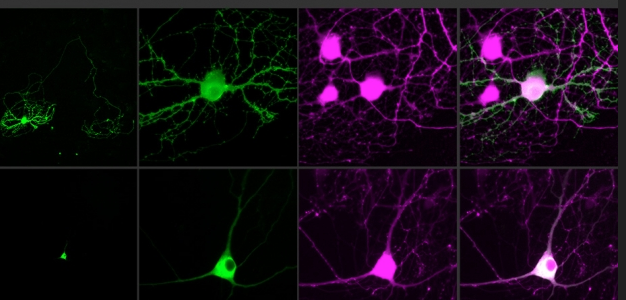
Controlling single neurons using optogenetics (credit: the researchers)
Researchers at MIT and Paris Descartes University have developed a technique for precisely mapping connections of individual neurons for the first time by triggering them with holographic laser light.
The technique is based on optogenetics (using light to stimulate or silence light-sensitive genetically modified protein molecules called “opsins” that are embedded in specific neurons). Current optogenetics techniques can’t isolate individual neurons (and their connections) because the light strikes a relatively large area — stimulating axons and dendrites of other neurons simultaneously (and these neurons may have different functions, even when nearby).
The new technique stimulates only the soma (body) of the neuron, not its connections. To achieve that, the researchers combined two new advances: an optimized holographic light-shaping microscope* and a localized, more powerful opsin protein called CoChR.
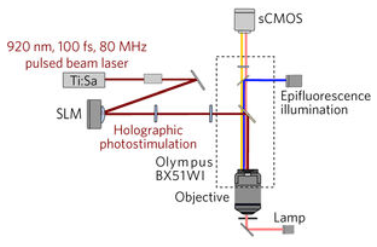
Two-photon computer-generated holography (CGH) was used to create three-dimensional sculptures of light that envelop only a target cell, using a conventional pulsed laser coupled with a widefield epifluorescence imaging system. (credit: Or A. Shemesh et al./Nature Nanoscience)
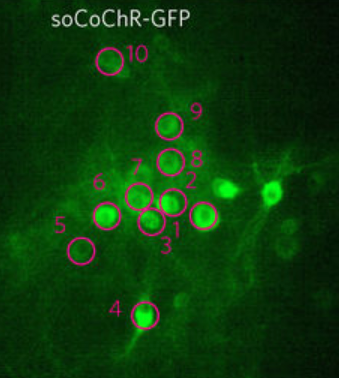
The researchers used an opsin protein called CoChR, which generates a very strong electric current in response to light, and fused it to a small protein that directs the opsin into the cell bodies of neurons and away from axons and dendrites, which extend from the neuron body, forming “somatic channelrhodopsin” (soCoChR). This new opsin enabled photostimulation of individual cells (regions of stimulation are highlighted by magenta circles) in mouse cortical brain slices with single-cell resolution and with less than 1 millisecond temporal (time) precision — achieving connectivity mapping on intact cortical circuits without crosstalk between neurons. (credit: Or A. Shemesh et al./Nature Nanoscience)
In the new study, by combining this approach with new ““somatic channelrhodopsin” opsins that cluster in the cell body, the researchers showed they could stimulate individual neurons with not only precise spatial control but also great control over the timing of the stimulation. When they target a specific neuron, it responds consistently every time, with variability that is less than one millisecond, even when the cell is stimulated many times in a row.
“For the first time ever, we can bring the precision of single-cell control toward the natural timescales of neural computation,” says Ed Boyden, an associate professor of brain and cognitive sciences and biological engineering at MIT, and a member of MIT’s Media Lab and McGovern Institute for Brain Research. Boyden is co-senior author with Valentina Emiliani, a research director at France’s National Center for Scientific Research (CNRS) and director of the Neurophotonics Laboratory at Paris Descartes University, of a study that appears in the Nov. 13 issue of Nature Neuroscience.
Mapping neural connections in real time
Using this technique, the researchers were able to stimulate single neurons in brain slices and then measure the responses from cells that are connected to that cell. This may pave the way for more precise diagramming of the connections of the brain, and analyzing how those connections change in real time as the brain performs a task or learns a new skill.
Optogenetics was co-developed in 2005 by Ed Boyden (credit: MIT)
One possible experiment, Boyden says, would be to stimulate neurons connected to each other to try to figure out if one is controlling the others or if they are all receiving input from a far-off controller.
“It’s an open question,” he says. “Is a given function being driven from afar, or is there a local circuit that governs the dynamics and spells out the exact chain of command within a circuit? If you can catch that chain of command in action and then use this technology to prove that that’s actually a causal link of events, that could help you explain how a sensation, or movement, or decision occurs.”
As a step toward that type of study, the researchers now plan to extend this approach into living animals. They are also working on improving their targeting molecules and developing high-current opsins that can silence neuron activity.
The research was funded by the National Institutes of Health, France’s National Research Agency, the Simons Foundation for the Social Brain, the Human Frontiers Science Program, John Doerr, the Open Philanthropy Project, the Howard Hughes Medical Institute, and the Defense Advanced Research Projects Agency.
* Traditional holography is based on reproducing, with light, the shape of a specific object, in the absence of that original object. This is achieved by creating an “interferogram” that contains the information needed to reconstruct an object that was previously illuminated by a reference beam. In computer-generated holography, the interferogram is calculated by a computer without the need of any original object. Combined with two-photon excitation, CGH can be used to refocus laser light to precisely illuminate a cell or a defined group of cells in the brain.
Abstract of Temporally precise single-cell-resolution optogenetics
Optogenetic control of individual neurons with high temporal precision within intact mammalian brain circuitry would enable powerful explorations of how neural circuits operate. Two-photon computer-generated holography enables precise sculpting of light and could in principle enable simultaneous illumination of many neurons in a network, with the requisite temporal precision to simulate accurate neural codes. We designed a high-efficacy soma-targeted opsin, finding that fusing the N-terminal 150 residues of kainate receptor subunit 2 (KA2) to the recently discovered high-photocurrent channelrhodopsin CoChR restricted expression of this opsin primarily to the cell body of mammalian cortical neurons. In combination with two-photon holographic stimulation, we found that this somatic CoChR (soCoChR) enabled photostimulation of individual cells in mouse cortical brain slices with single-cell resolution and <1-ms temporal precision. We used soCoChR to perform connectivity mapping on intact cortical circuits.