Master genetic switch for brain development discovered
November 24, 2015
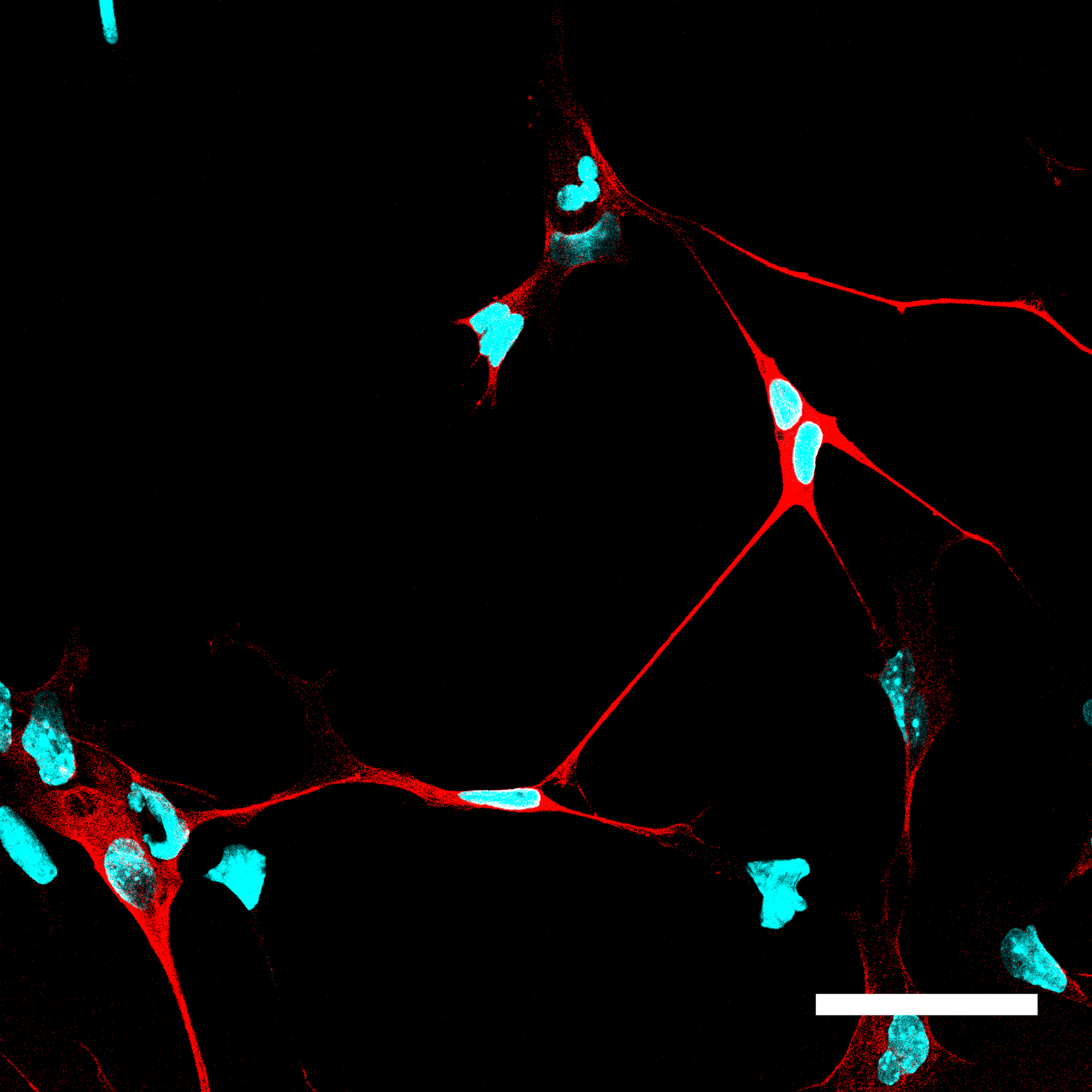
Cells in which NeuroD1 is turned on are reprogrammed to become neurons. Cell nuclei are shown in blue and neurons, with their characteristic long processes, are shown in red. (credit: A. Pataskar, J. Jung & V. Tiwari)
Scientists at the Institute of Molecular Biology (IMB) in Mainz, Germany have unraveled a complex regulatory mechanism that explains how a single gene, NeuroD1, can drive the formation of brain cells. The research, published in The EMBO Journal, is an important step towards a better understanding of how the brain develops and may lead to breakthroughs in regenerative medicine.
Neurodegenerative disorders, such as Parkinson’s disease, are often characterized by an irreversible loss of brain cells. Unlike many other cell types in the body, these neurons are generally not able to regenerate by themselves, so if the brain is damaged, it stays damaged. One hope of developing treatments for this kind of damage is to understand how the brain develops in the first place, and then try to imitate the process. However, the brain is also one of the most complex organs in the body, and very little is understood about the molecular pathways that guide its development.
An epigenetic memory
Scientists in Dr. Vijay Tiwari’s group at the Institute of Molecular Biology at Johannes Gutenberg University Mainz have been investigating a central gene in brain development, NeuroD1. This gene is expressed in the developing brain and marks the onset of neurogenesis (neuron growth).
In their research article, Tiwari and his colleagues have shown that during brain development, it also acts as a master regulator of a large number of genes that cause these cells to develop into neurons. They used a combination of neurobiology, epigenetics, and computational biology approaches to show that these genes are normally turned off in development, but NeuroD1 activity changes their epigenetic state in order to turn them on.
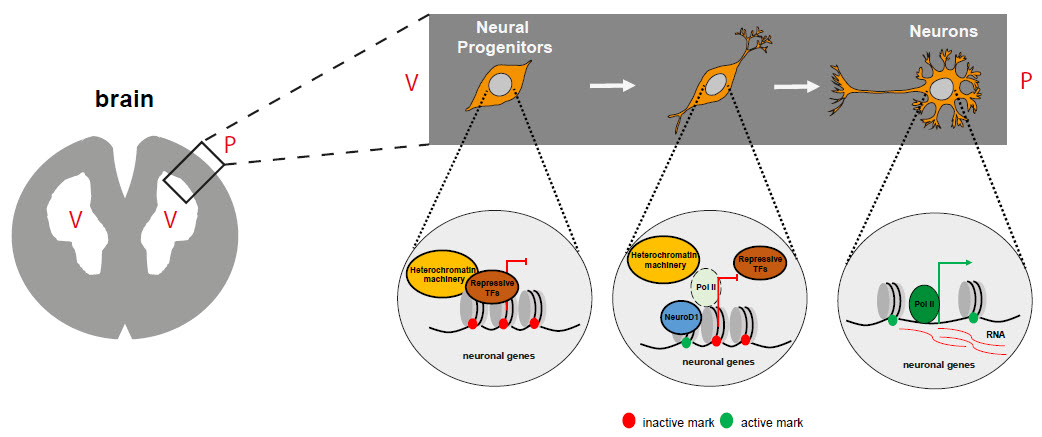
Diagram showing how NeuroD1 influences the development of neurons. During brain development, expression of NeuroD1 marks the onset of neurogenesis. NeuroD1 accomplishes this via epigenetic reprogramming: neuronal genes are switched on, and the cells develop into neurons. TF: transcription factor; V: ventricle; P: pial surface. (credit: A. Pataskar, J. Jung & V. Tiwari)
Strikingly, the researchers show that these genes remain switched on even after NeuroD1 is later switched off. They further show that this is because NeuroD1 activity leaves permanent epigenetic marks on these genes that keep them turned on. In other words, it creates an epigenetic memory of neuronal differentiation in the cell.
“This is a significant step towards understanding the relationship between DNA sequence, epigenetic changes, and cell fate,” says Tiwari. “It not only sheds new light on the formation of the brain during embryonic development but also opens up novel avenues for regenerative therapy.”
Abstract of NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program
Cell fate specification relies on the action of critical transcription factors that become available at distinct stages of embryonic development. One such factor is NeuroD1, which is essential for eliciting the neuronal development program and possesses the ability to reprogram other cell types into neurons. Given this capacity, it is important to understand its targets and the mechanism underlying neuronal specification. Here, we show that NeuroD1 directly binds regulatory elements of neuronal genes that are developmentally silenced by epigenetic mechanisms. This targeting is sufficient to initiate events that confer transcriptional competence, including reprogramming of transcription factor landscape, conversion of heterochromatin to euchromatin, and increased chromatin accessibility, indicating potential pioneer factor ability of NeuroD1. The transcriptional induction of neuronal fate genes is maintained via epigenetic memory despite a transient NeuroD1 induction during neurogenesis. NeuroD1 also induces genes involved in the epithelial‐to‐mesenchymal transition, thereby promoting neuronal migration. Our study not only reveals the NeuroD1‐dependent gene regulatory program driving neurogenesis but also increases our understanding of how cell fate specification during development involves a concerted action of transcription factors and epigenetic mechanisms.