Molecular homing beacon redirects human antibodies to fight threat of multidrug-resistant bacterial pathogens
May 7, 2015
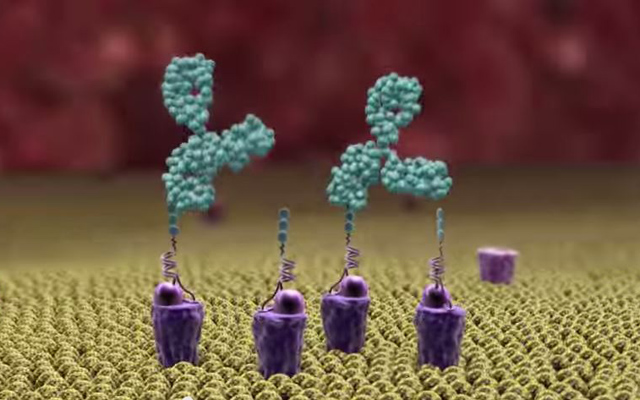
Alphamers (purple) topped with alpha-Gal (three green balls) act as homing beacons, attracting pre-existing anti-alpha-Gal antibodies (large green objects) to the bacteria (or other pathogen) surface (yellow), allowing human phagocytes in the body to attack it (credit: Altermune Technologies)
Good news on the serious threat of multidrug-resistant bacterial pathogens, which are rampant in hospitals and elsewhere: the Alphamer, a “molecular homing beacon,” has been invented by Nobel Laureate Kary Mullis, PhD, who previously invented polymerase chain reaction (PCR), a widely used lab technique for diagnostic tests, sequencing DNA, and other applications.
This entirely novel approach tags bacteria with a molecular “homing beacon” that attracts pre-existing antibodies in the body to attack the pathogens. Researchers at the University of California, San Diego School of Medicine and Skaggs School of Pharmacy and Pharmaceutical Sciences have reported preliminary success in testing the new method.
How the homing beacon works
One end of the homing beacon is made up of a DNA aptamer, a small piece of DNA that can be selected from a pool of billions of candidates based on its ability to bind tightly to a particular bacterial or other target. In the current test case, the aptamer specifically targeted group A Streptococcus, the bacteria that causes strep throat and invasive skin infections, while leaving human cells untouched.
The other end of the homing beacon is alpha-Gal, a type of sugar molecule. Humans naturally produce antibodies against alpha-Gal. That’s because alpha-Gal is foreign to humans. Other mammals and some microbes produce it. Humans have evolved antibodies against it whenever we have eaten meat or have been exposed to alpha-Gal-generating microbes in our environment.
To test the homing beacon — or “Alphamer” — against live strep bacteria, Mullis enlisted the help of Victor Nizet, MD, professor of pediatrics and pharmacy at UC San Diego, whose laboratory studies how pathogens interact with the human immune system. The research team found that Alphamers not only bind strep and recruit anti-Gal antibodies to the bacterial surface, they also helps human immune cells engulf and kill the Alphamer-coated bacteria.
The study offers the first proof-of-concept that Alphamers have the potential to specifically redirect pre-existing antibodies to bacteria and rapidly activate an antibacterial immune response.
MRSA is next target
“Our next step is to test Alphamers in animal models of infection with multidrug-resistant bacteria that pose a public health threat, such as MRSA,” said first author Sascha Kristian, PhD, visiting research scholar at UC San Diego and associate research director at Altermune Technologies, a company Mullis founded to develop Alphamers into unique therapeutics. “Meanwhile, we’ll also be tweaking the Alphamer to make it more potent and more resistant to degradation by the body.”
If Alphamers continue to show promise, researchers might be able to apply the same concept to attack any type of bacteria or virus, or perhaps even cancer cells, the authors suggest in a paper published in the Journal of Molecular Medicine.
“We’re picturing a future in which doctors have a case full of pathogen-specific Alphamers at their disposal,” Nizet said. “They see an infected patient, identify the causative bacteria, and pull out the appropriate alphamer to instantly enlist the support of the immune system in curing the infection.”
“The emergence and rapid spread of multi-drug resistant (MDR) bacteria represent a severe threat to the global health and economy,” the authors note. In 2012, the Director-General of the World Health Organization, Dr. Margaret Chan, warned that we risk entering a “post-antibiotic era,” which “means, in effect, an end to modern medicine as we know it.”
According to a recent U.S. Centers for Disease Control and Prevention (CDC) report, in the U.S. alone, an estimated two million people develop serious MDR bacterial infections annually, resulting in at least 23,000 deaths.
Altermune | Alphamers
Abstract of Retargeting pre-existing human antibodies to a bacterial pathogen with an alpha-Gal conjugated aptamer
The ever-increasing threat of multi-drug resistant bacterial infections has spurred renewed interest in alternative approaches to classical antibiotic therapy. In contrast to other mammals, humans do not express the galactose-α-1,3-galactosyl-β-1,4-N-acetyl-glucosamine (α-Gal) epitope. As a result of exposure of humans to α-Gal in the environment, a large proportion of circulating antibodies are specific for the trisaccharide. In this study, we examine whether these anti-Gal antibodies can be recruited and redirected to exert anti-bacterial activity. We show that a specific DNA aptamer conjugated to an α-Gal epitope at its 5′ end, herein termed an alphamer, can bind to group AStreptococcus (GAS) bacteria by recognition of a conserved region of the surface-anchored M protein. The anti-GAS alphamer was shown to recruit anti-Gal antibodies to the streptococcal surface in an α-Gal-specific manner, elicit uptake and killing of the bacteria by human phagocytes, and slow growth of invasive GAS in human whole blood. These studies provide a first in vitro proof of concept that alphamers have the potential to redirect pre-existing antibodies to bacteria in a specific manner and trigger an immediate antibacterial immune response. Further validation of this novel therapeutic approach of applying α-Gal technology in in vivo models of bacterial infection is warranted.