Nanoparticle ‘cluster bombs’ destroy cancer cells
March 30, 2016
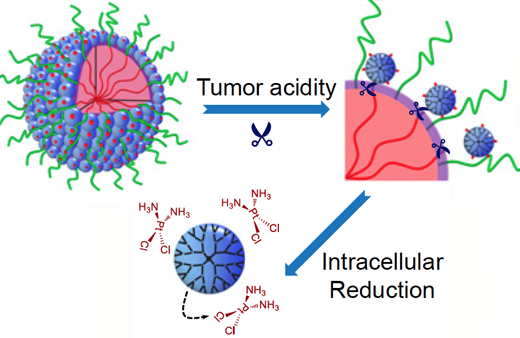
The nanoparticles start out relatively large (100 nm) (large blue circle, upper left) to enable smooth transport into the tumor through leaky blood vessels. Then, in acidic conditions found close to tumors, the particles discharge “bomblets” (right, small blue circles) just 5 nm in size. Once inside tumor cells, a second chemical step activates the platinum-based drug cisplatin (bottom) to attack the cancer directly. (credit: Emory Health Sciences)
Scientists have devised a triple-stage stealth “cluster bomb” system for delivering the anti-cancer chemotherapy drug cisplatin, using nanoparticles designed to break up when they reach a tumor:
- The nanoparticles start out relatively large — 100 nanometers wide — so that they can move through the bloodstream and smoothly transport into the tumor through leaky blood vessels.
- As they detect acidic conditions close to tumors, the nanoparticles discharge “bomblets” just 5 nanometers in size to penetrate tumor cells.
- Once inside tumor cells, the bomblets release the platinum-based cisplatin, which kills by crosslinking and damaging DNA.
Doctors have used cisplatin to fight several types of cancer for decades, but toxic side effects — to the kidneys, nerves and inner ear — have limited its effectiveness. But in research with three different mouse tumor models*, the researchers have now shown that their nanoparticles can enhance cisplatin drug accumulation in tumor tissues for several types of cancer.
Details of the research — by teams led by professor Jun Wang, PhD, at the University of Science and Technology of China and by professor Shuming Nie, PhD, in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory — were published this week in the journal PNAS.
* When mice bearing human pancreatic tumors were given the same doses of free cisplatin or cisplatin clothed in pH-sensitive nanoparticles, the level of platinum in tumor tissues was seven times higher with the nanoparticles. This suggests the possibility that nanoparticle delivery of a limited dose of cisplatin could restrain the toxic side effects during cancer treatment.
The researchers also showed that the nanoparticles were effective against a cisplatin-resistant lung cancer model and an invasive metastatic breast cancer model in mice. In the lung cancer model, a dose of free cisplatin yielded just 10 percent growth inhibition, while the same dose clothed in nanoparticles yielded 95 percent growth inhibition, the researchers report. In the metastatic breast cancer model, treating mice with cisplatin clothed in nanoparticles prolonged animal survival by weeks; 50 percent of the mice were surviving at 54 days with nanoparticles compared with 37 days for the same dose of free cisplatin.
Abstract of Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy
A principal goal of cancer nanomedicine is to deliver therapeutics effectively to cancer cells within solid tumors. However, there are a series of biological barriers that impede nanomedicine from reaching target cells. Here, we report a stimuli-responsive clustered nanoparticle to systematically overcome these multiple barriers by sequentially responding to the endogenous attributes of the tumor microenvironment. The smart polymeric clustered nanoparticle (iCluster) has an initial size of ∼100 nm, which is favorable for long blood circulation and high propensity of extravasation through tumor vascular fenestrations. Once iCluster accumulates at tumor sites, the intrinsic tumor extracellular acidity would trigger the discharge of platinum prodrug-conjugated poly(amidoamine) dendrimers (diameter ∼5 nm). Such a structural alteration greatly facilitates tumor penetration and cell internalization of the therapeutics. The internalized dendrimer prodrugs are further reduced intracellularly to release cisplatin to kill cancer cells. The superior in vivo antitumor activities of iCluster are validated in varying intractable tumor models including poorly permeable pancreatic cancer, drug-resistant cancer, and metastatic cancer, demonstrating its versatility and broad applicability.