New chemical method could revolutionize graphene use in electronics
June 16, 2017
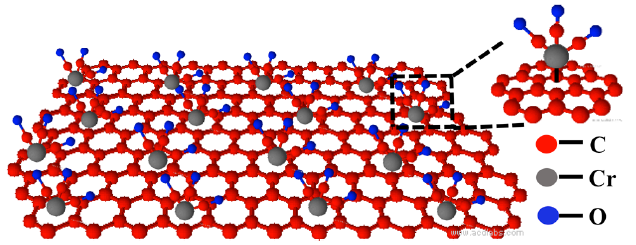
Adding a molecular structure containing carbon, chromium, and oxygen atoms retains graphene’s superior conductive properties. The metal atoms (silver, in this experiment) to be bonded are then added to the oxygen atoms on top. (credit: Songwei Che et al./Nano Letters)
University of Illinois at Chicago scientists have solved a fundamental problem that has held back the use of wonder material graphene in a wide variety of electronics applications.
When graphene is bonded (attached) to metal atoms (such as molybdenum) in devices such as solar cells, graphene’s superior conduction properties degrade.
The solution: Instead of adding molecules directly to the individual carbon atoms of graphene, the new method first adds a sort of buffer (consisting of chromium, carbon, and oxygen atoms) to the graphene, and then adds the metal atoms to this buffer material instead. That enables the graphene to retain its unique properties of electrical conduction.
In an experiment, the researchers successfully added silver nanoparticles to graphene with this method. That increased the material’s ability to boost the efficiency of graphene-based solar cells by 11 fold, said Vikas Berry, associate professor and department head of chemical engineering and senior author of a paper on the research, published in Nano Letters.
Researchers at Indian Institute of Technology and Clemson University were also involved in the study. The research was funded by the National Science Foundation.
Abstract of Retained Carrier-Mobility and Enhanced Plasmonic-Photovoltaics of Graphene via ring-centered η6 Functionalization and Nanointerfacing
Binding graphene with auxiliary nanoparticles for plasmonics, photovoltaics, and/or optoelectronics, while retaining the trigonal-planar bonding of sp2 hybridized carbons to maintain its carrier-mobility, has remained a challenge. The conventional nanoparticle-incorporation route for graphene is to create nucleation/attachment sites via “carbon-centered” covalent functionalization, which changes the local hybridization of carbon atoms from trigonal-planar sp2to tetrahedral sp3. This disrupts the lattice planarity of graphene, thus dramatically deteriorating its mobility and innate superior properties. Here, we show large-area, vapor-phase, “ring-centered” hexahapto (η6) functionalization of graphene to create nucleation-sites for silver nanoparticles (AgNPs) without disrupting its sp2 character. This is achieved by the grafting of chromium tricarbonyl [Cr(CO)3] with all six carbon atoms (sigma-bonding) in the benzenoid ring on graphene to form an (η6-graphene)Cr(CO)3 complex. This nondestructive functionalization preserves the lattice continuum with a retention in charge carrier mobility (9% increase at 10 K); with AgNPs attached on graphene/n-Si solar cells, we report an ∼11-fold plasmonic-enhancement in the power conversion efficiency (1.24%).