New hope for sufferers of degenerative muscle disorders
November 27, 2012
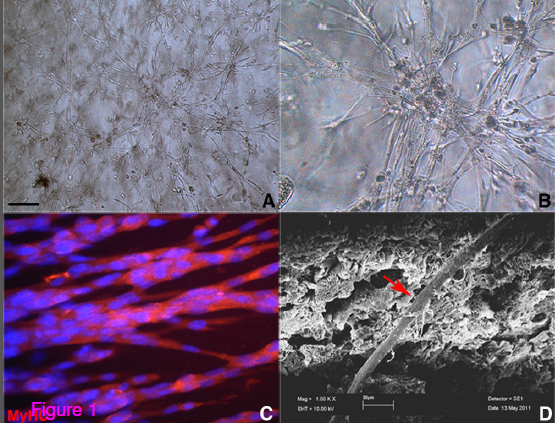
Mesoangioblast stem cells cultured in polyethylene glycol-fibrinogen (PF) hydrogels. (A,B) Phase-contrast images of mesoangioblasts in hydrogel, giving rise to a robust three-dimensional myofiber network. (C) Immunofluorescence showing multinucleated muscle fibers. (D) Scanning electron microscopy image revealing the presence of differentiating skeletalmuscle fibers (red arrows) within the PF hydrogel. Scale bar: (A) 200 μm, (B) 50 μm and (C) 10 μm. (Credit: Claudia Fuoco et al./Skeletal Muscle)
A new therapeutic technique to repair and rebuild muscle for sufferers of degenerative muscle disorders has been developed by an international team of researchers, according to a study published today in BioMed Central’s open access journal Skeletal Muscle.
The therapy brings together two existing techniques for muscle repair — cell transplantation (mesoangioblast stem cells) and tissue engineering, delivering the stem cells via a hydrogel cell-carrier matrix.
A number of conditions can lead to considerable degeneration or loss of skeletal muscle and, since skeletal muscle has a limited capacity for self repair, so therapies for muscle reconstruction or regeneration are often necessary.
There are currently two ways to rebuild muscle: cell transplantation, whereby stem cells are injected directly into the muscle or arteries, and tissue engineering, whereby cells are embedded on a biomaterial scaffold to reconstruct a whole muscle.
Stem cell transplantation on its own can be limited by poor cell survival, but the authors hoped that the technique in combination with tissue engineering could increase the chances of efficacy for localized disorders of muscle.
The research team, comprised of researchers from institutions all over Europe, embedded mesoangioblast (Mab) cells within a polyethylene glycol and fibrinogen (PF) hydrogel scaffold that has a proven track record in tissue and cardiac engineering. The Mab/PF grafts were then injected into mice, directly into the chronically inflamed and sclerotic regions typical of the advanced stages of muscular dystrophy. The team observed increased engraftment and survival of Mabs when injected with PF than with Mabs suspended in saline solution.
Five weeks after treatment, analyses revealed that Mabs had better integrated into regenerating muscle fibers when used with a PF carrier than when used without. In addition, there was better organization of muscle fibers when Mabs was used in combination with PF.
“This study demonstrates a novel tissue engineering approach that is capable of producing enriched donor cell engraftment into skeletal muscle after an acute injury or in more-difficult-to-treat advanced-stage muscular dystrophy,” lead author Cesare Gargioli commented.
“Both Mabs and PF are currently undergoing separate clinical trials, but their combined use may increase efficacy for sufferers with more localized forms of muscular dystrophy or disorders that lead to damage of skeletal muscle, including hernias and sphincter disorders.”