New hydrogel improves delivery of breast-cancer treatment
November 6, 2013
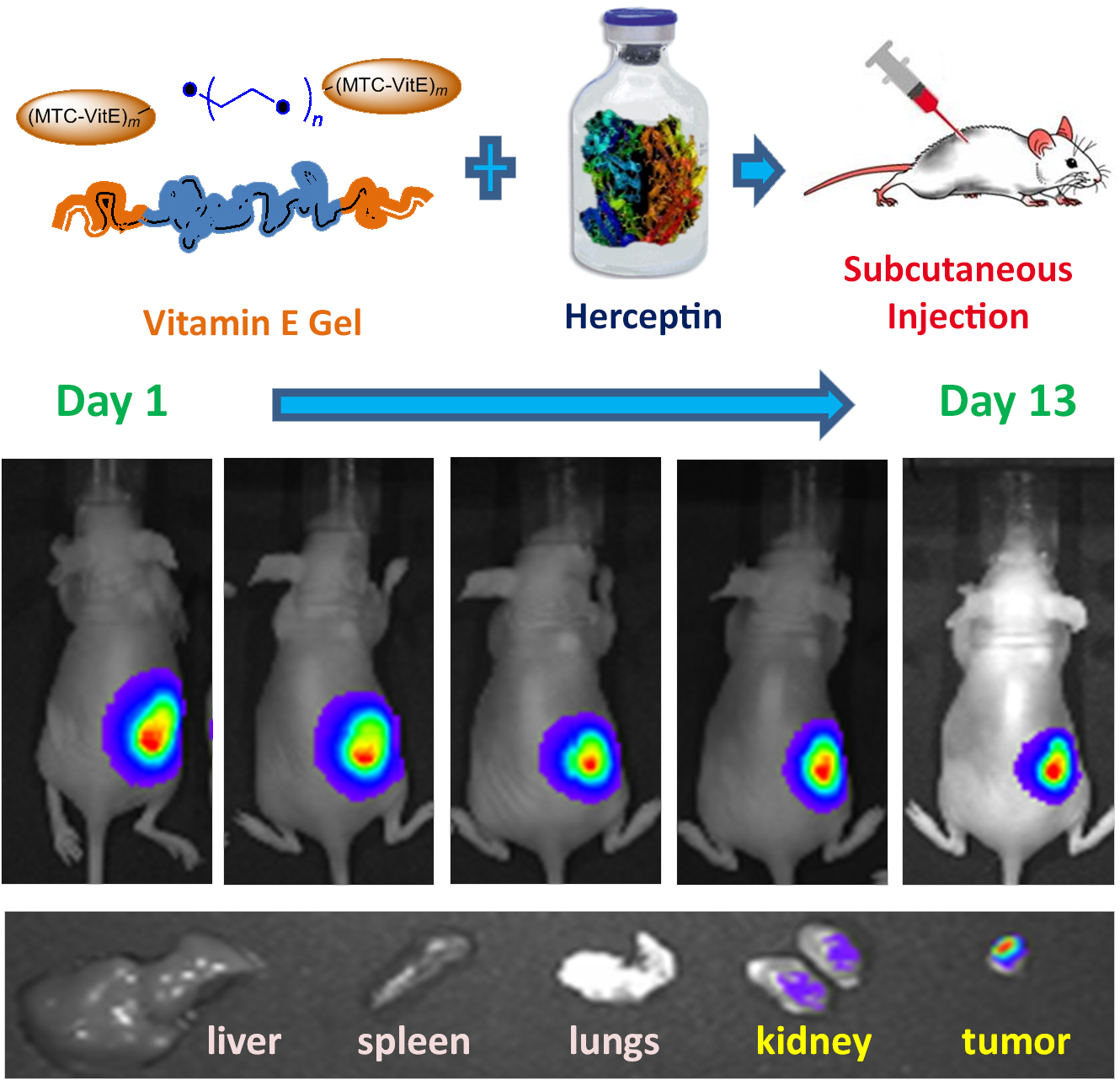
Herceptin was loaded into Vitamin E gel by convenient mixing. The Herceptin-loaded hydrogel was tested in mice bearing breast cancer. Herceptin was preferably accumulated in the tumor tissues as compared to the healthy animal organs. (Credit: IBN)
The Institute of Bioengineering and Nanotechnology (IBN) in Singapore and IBM Research (IBM) have developed a new non-toxic hydrogel that is capable of shrinking breast cancer tumors more rapidly than existing therapies.
As described in their publication in Advanced Functional Materials, the Vitamin E-incorporated hydrogel can be easily injected under the skin without causing any inflammatory response, and releases anti-cancer drugs in a sustained manner over several weeks. This reduces the need for frequent drug administration, paving the way for the tumors to be eradicated in fewer treatments.
Breast cancer is the most common invasive cancer affecting women worldwide, including in Singapore. One in four breast cancer patients will have a significantly lower survival rate as they possess a particularly vicious type of cancer gene known as the human epidermal growth factor receptor 2 (HER2+), which causes rapid, unrestrained growth and division of cells in their breasts.
Herceptin, a US Food and Drug Administration approved therapeutic for the treatment of HER2+, helps to combat this type of cancer by regulating the cancer growth. Currently, this drug is administered intravenously in most clinics on a weekly basis, with each treatment session lasting 30 to 90 minutes. The need for frequent infusion of Herceptin and the accompanying discomfort may affect patient compliance adversely.
So IBN and IBM researchers aimed to reduce the number of injections and injection time required by creating a biocompatible, biodegradable and injectable hydrogel that can be conveniently injected into the body and release Herceptin in a sustained manner. Through this, they were able to reduce the frequency of drug administration from weekly to only once in four weeks.
Recent clinical trials using subcutaneous injection of Herceptin shortened the injection time to around 5 minutes and were reported to have comparable therapeutic efficacy as traditional methods at the same dosing schedule.
The new drug administration method via IBN’s and IBM’s hydrogel offers a further improvement on these studies as it supplies Herceptin continuously over a prolonged period of time. It also helped to shrink the tumors over fewer administrations. In animal studies with tumor-bearing mice, the tumors shrank in size by 77% 28 days after the Herceptin-loaded hydrogel was injected subcutaneously. The hydrogel did not evoke any chronic inflammatory response and degraded within 6 weeks post-administration.
The IBN and IBM scientists have filed a patent on their hydrogel technology and would like to engage pharmaceutical companies to further develop the hydrogel for future clinical applications. Specifically, the researchers would like to use their hydrogel formulation to deliver antibodies subcutaneously to improve disease treatment efficacy and patient compliance.
Abstract of Advanced Functional Materials paper
In this study, ‘ABA’-type triblock copolymers of vitamin E-functionalized polycarbonate and poly(ethylene glycol), i.e., VitEm-PEG-VitEm, with extremely short hydrophobic block VitEm, are synthesized and employed to form physically cross-linked injectable hydrogels for local and sustained delivery of Herceptin. The hydrogels are formed at low concentrations (4–8 wt%). By varying polymer composition and concentration, the rheological behavior, porosity, and drug release properties of hydrogels are readily tunable. The in vitro antitumor specificity and efficacy of Herceptin in hydrogel and solution are investigated by MTT assay against normal and human breast cancer cell lines with different HER2 expression levels. The results demonstrate that the Herceptin-loaded hydrogel is specific towards HER2-overexpressing cancer cells and cytotoxic action is comparable to that of the Herceptin solution. The biocompatibility and biodegradability of hydrogel are evaluated in mice with subcutaneous injection by histological examination. It is observed that the hydrogel does not evoke a chronic inflammatory response and degrades within 6 weeks post administration. Biodistribution and anti-tumor efficacy studies performed in BT474 tumor-bearing mice show that single subcutaneous injection of Herceptin-loaded hydrogel at a site close to the tumor enhances the retention of the antibody within the tumor. This leads to superior anti-tumor efficacy as compared to intravenous (i.v.) and subcutaneous (s.c.) delivery of Herceptin in solution. The tumor size shrank by 77% at Day 28. When the hydrogel is injected at a distal location away from the tumor site, anti-tumor efficacy is similar to that of weekly i.v. injections of Herceptin solution over 4 weeks, with the number of injections reduced from 4 to 1. These findings suggest that this hydrogel has great potential for use in subcutaneous and sustained delivery of antibodies to increase therapeutic efficacy and/or improve patient compliance.
Dr Yi Yan Yang, Group Leader, Institute of Bioengineering and Nanotechnology:
1. What are the practical uses of this innovation?
This technology can be used to deliver a range of therapeutics, including small molecular (anticancer drugs or antibiotics) and macromolecular (mAbs) drugs, in a sustained release manner. The hydrogel formulation can be administered via injection or topically.
Sustained release of therapeutics is expected to provide better efficacy and improve patient compliance.
2. When can we expect this innovation to be available commercially?
We are looking for partners that can help advance the technology for final clinical application.
3. How does this innovation compare to others?
This hydrogel has unique rheological or flow property that allows it to be transformed from a gel to a liquid via shear stress, i.e. enabling injection via a syringe, it causes minimal immune response, allows sustained delivery and degrades after use. It is made of polymer at lower concentrations than other hydrogel technologies due to the presence of hydrophobic vitamin E.