New nanodevice defeats drug resistance and releases cancer drugs
March 6, 2015
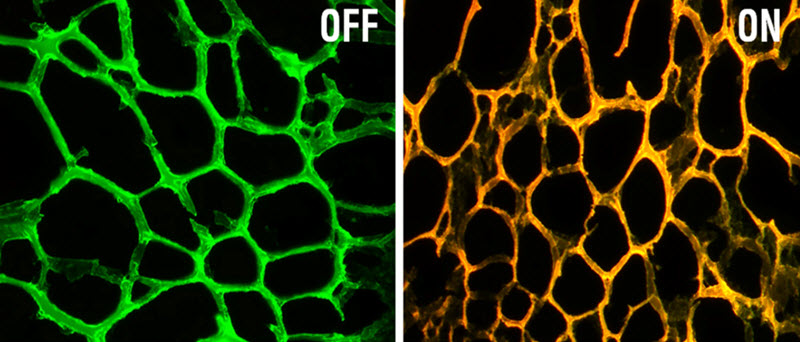
At left is the new nanodevice, consisting of a hydrogel embedded with gold nanoparticles coated with DNA that targets a gene called MRP-1. At right, the device fluoresces after encountering the target gene sequence. (credit: MIT)
A new nanodevice developed by MIT researchers can help overcome cancer cell drug resistance (after chemotherapy) by first blocking the gene that confers drug resistance, then launching a new chemotherapy attack against the disarmed tumors.
The device, which consists of gold nanoparticles embedded in a hydrogel that can be injected or implanted at a tumor site, could also be used more broadly to disrupt any gene involved in cancer.
A gold nanoparticle embedded in a hydrogel (gray) and coated with a “nanobeacon” DNA hairpin (blue), near-infrared dye (red and green) and a dark-quencher (black). The nanoparticle can be injected or implanted at a tumor site to disrupt any gene involved in cancer. (credit: João Conde, Nuria Oliva, and Natalie Artzi/PNAS)
“You can target any genetic marker and deliver a drug, including those that don’t necessarily involve drug-resistance pathways. It’s a universal platform for dual therapy,” says Natalie Artzi, a research scientist at MIT’s Institute for Medical Engineering and Science (IMES), an assistant professor at Harvard Medical School, and senior author of a paper describing the device in the Proceedings of the National Academy of Sciences.
To demonstrate the effectiveness of the new approach, Artzi and colleagues tested it in mice implanted with a type of human breast tumor known as a triple negative tumor. Such tumors, which lack any of the three most common breast cancer markers — estrogen receptor, progesterone receptor, and Her2 — are usually very difficult to treat.
Using the new device to block the gene for multidrug resistant protein 1 (MRP1) and then deliver the chemotherapy drug 5-fluorouracil, the researchers were able to shrink tumors by 90 percent in two weeks.
Overcoming resistance
“Drug resistance is a huge hurdle in cancer therapy and the reason why chemotherapy, in many cases, is not very effective”, says João Conde, an IMES postdoc and lead author of the PNAS paper.
To overcome this, the researchers created gold nanoparticles coated with strands of DNA complementary to the sequence of MRP1 messenger RNA — the snippet of genetic material that carries DNA’s instructions to the rest of the cell.
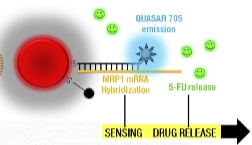
Sensing target mRNA sequence and drug release (credit: João Conde, Nuria Oliva, and Natalie Artzi/PNAS)
These strands of DNA, which the researchers call “nanobeacons,” fold back on themselves to form a closed hairpin structure.
However, when the DNA encounters the correct mRNA sequence inside a cancer cell, it unfolds and binds to the mRNA, preventing it from generating more molecules of the MRP1 protein. As the DNA unfolds, it also releases molecules of 5-fluorouracil that were embedded in the strand.
This drug then attacks the tumor cell’s DNA, since MRP1 is no longer around to pump it out of the cell.
“When we silence the gene, the cell is no longer resistant to that drug, so we can deliver the drug that now regains its efficacy,” Conde says.
When each of these events occurs — sensing the MRP1 protein and releasing 5-fluorouracil — the device emits fluorescence of different wavelengths, allowing the researchers to visualize what is happening inside the cells. Because of this, the particles could also be used for diagnosis — determining if a certain cancer-related gene is activated in tumor cells.
Controlled drug release
The DNA-coated gold nanoparticles are embedded in an adhesive gel that stays in place and coats the tumor after being implanted. This local administration of the particles protects them from degradation that might occur if they were administered throughout the body, and also enables sustained drug release, Artzi says.
In their mouse studies, the researchers found that the particles could silence MRP1 for up to two weeks, with continuous drug release over that time, effectively shrinking tumors.
This approach could be adapted to deliver any kind of drug or gene therapy targeted to a specific gene involved in cancer, the researchers say. They are now working on using it to silence a gene that stimulates gastric tumors to metastasize to the lungs.
“This is an impressive study that harnesses expertise at the interface of materials science, nanotechnology, biology, and medicine to enhance the efficacy of traditional chemotherapeutics,” says Jeffrey Karp, an associate professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, who was not involved in the research. “Hopefully this approach will perform in studies beyond 14 days and be translatable to patients, who are desperate for new and more effective treatment regimens.”
Graduate student Nuria Oliva is also an author of the paper. The research was funded by the National Cancer Institute and a Marie Curie International Outgoing Fellowship.
Abstract of Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance
Multidrug resistance (MDR) in cancer cells is a substantial limitation to the success of chemotherapy. Here, we describe facile means to overcome resistance by silencing the multidrug resistance protein 1 (MRP1), before chemotherapeutic drug delivery in vivo with a single local application. Our platform contains hydrogel embedded with dark-gold nanoparticles modified with 5-fluorouracil (5-FU)-intercalated nanobeacons that serve as an ON/OFF molecular nanoswitch triggered by the increased MRP1 expression within the tumor tissue microenvironment. This nanoswitch can sense and overcome MDR prior to local drug release. The nanobeacons comprise a 5-FU intercalated DNA hairpin, which is labeled with a near-infrared (NIR) dye and a dark-quencher. The nanobeacons are designed to open and release the intercalated drug only upon hybridization of the DNA hairpin to a complementary target, an event that restores fluorescence emission due to nanobeacons conformational reorganization. Despite the cross-resistance to 5-FU, more than 90% tumor reduction is achieved in vivo in a triple-negative breast cancer model following 80% MRP1 silencing compared with the continuous tumor growth following only drug or nanobeacon administration. Our approach can be applied to reverse cross-resistance to other chemotherapeutic drugs and restore treatment efficacy. As a universal nanotheranostic probe, this platform can pave the way to early cancer detection and treatment.