New synthesized molecule could reduce brain damage in stroke victims
March 14, 2016
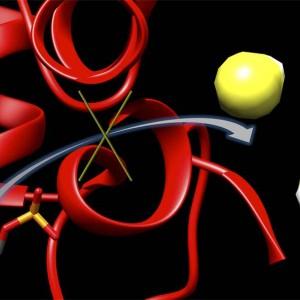
This graphic depicts a new inhibitor, 6S, locking up an enzyme (red) to block the production of hydrogen sulfide (yellow and white). Hydrogen sulfide concentrations have been shown to climb after the onset of a stroke, leading to brain damage. (credit: Matthew Beio, University of Nebraska-Lincoln)
A new molecule known as 6S has reduced the death of brain tissue from ischemic stroke by up to 66 percent in rats while reducing the accompaning inflammation, researchers at the University of Nebraska-Lincoln and the National University of Singapore reported March 9 in an open-access paper published by the journal ACS Central Science.
The inhibitor molecule works by binding to cystathionine beta-synthase (CBS), an enzyme that normally helps regulate cellular function, but can also trigger production of toxic levels of hydrogen sulfide in the brain. (That buildup initiates brain damage after strokes by disrupting blood flow, which prevents oxygen and glucose from reaching brain tissue, ultimately killing neurons and other cells.)
The researchers modeled the inhibitor on a naturally occurring molecule produced by the CBS enzyme, tailoring the molecule’s structure to improve its performance.* That increased the inhibitor’s binding time from less than a second to hours.
Because the 6S inhibitor has only demonstrated its effects in cell cultures and the brain tissue of rats, the researchers cautioned that it represents just an initial step toward developing a stroke-treating drug for humans.
Research and facilities that contributed to the study were partly funded by the American Heart Association, the National Science Foundation, and the National Institutes of Health.
The World Health Organization has estimated that stroke kills more than 6 million people annually.
* The researchers replaced functional groups of atoms known as amines with hydrazines.
Abstract of “Zipped Synthesis” by Cross-Metathesis Provides a Cystathionine β-Synthase Inhibitor that Attenuates Cellular H2S Levels and Reduces Neuronal Infarction in a Rat Ischemic Stroke Model
The gaseous neuromodulator H2S is associated with neuronal cell death pursuant to cerebral ischemia. As cystathionine β-synthase (CBS) is the primary mediator of H2S biogenesis in the brain, it has emerged as a potential target for the treatment of stroke. Herein, a “zipped” approach by alkene cross-metathesis into CBS inhibitor candidate synthesis is demonstrated. The inhibitors are modeled after the pseudo-C2-symmetric CBS product (l,l)-cystathionine. The “zipped” concept means only half of the inhibitor needs be constructed; the two halves are then fused by olefin cross-metathesis. Inhibitor design is also mechanism-based, exploiting the favorable kinetics associated with hydrazine-imine interchange as opposed to the usual imine–imine interchange. It is demonstrated that the most potent “zipped” inhibitor 6S reduces H2S production in SH-SY5Y cells overexpressing CBS, thereby reducing cell death. Most importantly, CBS inhibitor 6S dramatically reduces infarct volume (1 h post-stroke treatment; ∼70% reduction) in a rat transient middle cerebral artery occlusion model for ischemia.