Noninvasive retinal imaging device detects Alzheimer’s 20 years in advance
July 22, 2014
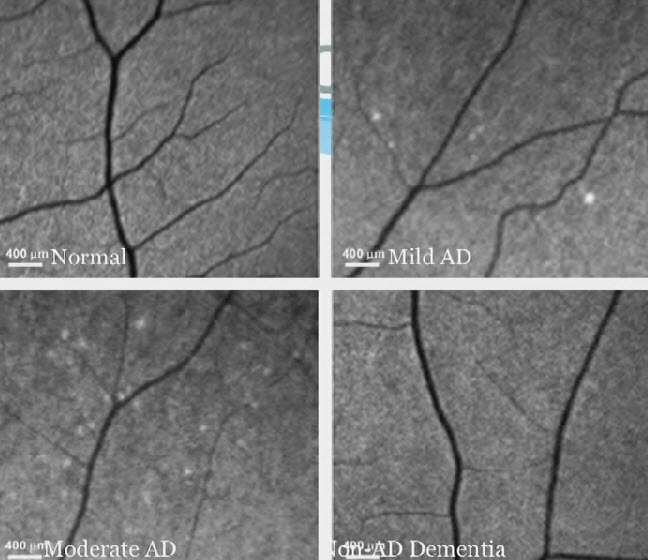
Retina test for future Alzheimer’s disease (AD), showing plaques. Upper left: normal retina.Upper right: mild AD. Lower left: Moderate AD. Lower right: Non-AD dementia. (Credit: Keith Black, MD)
Cedars-Sinai Medical Center researchers have developed a noninvasive retinal imaging device that can provide early detection of changes indicating Alzheimer’s disease 15 to 20 years before clinical diagnosis.
“In preliminary results in 40 patients, the test could differentiate between Alzheimer’s disease and non-Alzheimer’s disease with 100 percent sensitivity and 80.6 percent specificity, meaning that all people with the disease tested positive and most of the people without the disease tested negative,” said Shaun Frost, a biomedical scientist and the study manager at the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Australia’s national science agency.
Keith Black, MD, professor and chair of Cedars-Sinai’s Department of Neurosurgery and director of the Maxine Dunitz Neurosurgical Institute and the Ruth and Lawrence Harvey Chair in Neuroscience, said the accumulation of beta-amyloid plaque in the brain is a hallmark sign of Alzheimer’s, but current tests detect changes only after the disease has advanced to late stages.
Google | Solve for X: Keith Black on Alzheimer’s diagnostics
Researchers believe that as treatment options improve, early detection will be critical, but existing diagnostic methods are inconvenient, costly and impractical for routine screening.
“PET scans require the use of radioactive tracers, and cerebrospinal fluid analysis requires that patients undergo invasive and often painful lumbar punctures, but neither approach is quite feasible, especially for patients in the earlier stages of disease,” he said. Positron emission tomography, or PET, is the current diagnostic standard.
“A few years ago, we discovered at Cedars-Sinai that the plaques associated with Alzheimer’s disease occur not only in the brain but also in the retina.”
Common curcumin spice marks plaque in the retina
“By ‘staining’ the plaque with curcumin, a component of the common spice turmeric, we could detect it in the retina even before it began to accumulate in the brain. The device we developed enables us to look through the eye — just as an ophthalmologist looks through the eye to diagnose retinal disease — and see these changes.”
This clinical trial was designed to enable researchers to correlate retinal plaque detected by optical imaging with brain plaque detected by PET scans. Studies involved three groups: patients diagnosed with Alzheimer’s, a group with mild cognitive impairment, and a group of people with no evidence of brain abnormality.
“This large double-blind clinical trial appears to validate our novel human retinal amyloid imaging approach using curcumin labeling. It further demonstrates significant correlation with brain amyloid burden, thereby predicting accumulation of plaques in the brain through the retina,” said Koronyo-Hamaoui, a faculty principal investigator and head of the Neuroimmunology and Retinal Imaging Laboratory at Cedars-Sinai.
FDA approval in 2015 sought
One goal of the research is to “detect the changes of Alzheimer’s disease as early as possible so that as treatment approaches improve we will be able to provide interventions before cognitive deficits appear — in other words, before too much irreversible damage has been done,” Black explained to KurzweilAI in an email interview.
“A second goal is to develop a method for monitoring the effectiveness of treatments. We believe retinal imaging may make both of these goals possible. The retina is actually part of the central nervous system, and we have shown that Alzheimer’s-associated amyloid accumulates in the retina even before it affects the brain. This appears to give us a way to predict Alzheimer’s changes in the brain before they occur, and it may give us a simple and cost-effective way to gauge disease progression and treatment benefit.
“We are aiming for FDA approval in 2015.”
The optical-imaging technology has been licensed by Cedars-Sinai to NeuroVision Imaging LLC for further development. “Potentially, this could be a 20-minute test which you could get when you go to your ophthalmologist for your regular eye screening,” Black, who is chairman of the company, said in the video.
The retinal imaging study is being conducted as part of the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing, often referred to as AIBL, a longitudinal research cohort with more than 1,300 subjects. It is a collaborative effort of Austin Health, the CSIRO, Edith Cowan University, the Florey Institute of Neuroscience and Mental Health and the National Ageing Research Institute, with NeuroVision Imaging LLC providing image processing, and data collection and analysis support for the retinal imaging substudy.
The researchers presented their findings July 15 at the Alzheimer’s Association International Conference 2014 in Copenhagen.