Overuse and misuse of antibiotics leading to rise of resistant strains of pathogenic bacteria
November 15, 2016
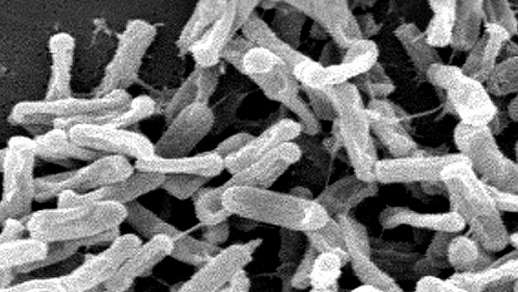
Prolonged preventive antibiotics after surgical incision closure do not prevent infections, but they do change the composition of bacteria in the host at other anatomic locations. The result is resistant colonization of the patient and potentially an infection with Clostridium difficile bacteria, shown here. (credit: CDC)
Overuse and misuse of antimicrobial agents in hospitals is an urgent problem. Surgeons around the world, who often prescribe antibiotics for surgical prophylaxis, need to take a leadership role in promoting “antimicrobial stewardship programs” (ASPs) that can optimize antimicrobial agent use in the hospital.
That’s the message from the Surgical Infection Society and the World Society of Emergency Surgery in an open-access paper entitled “Antimicrobial Stewardship: A Call to Action for Surgeons,” published in Surgical Infections, a peer-reviewed journal published today (Tuesday, Nov. 15) online ahead of print by Mary Ann Liebert, Inc.*
The authors emphasize the urgent need to standardize the use of antimicrobial agents. Approximately 15% of antibiotic prescribing in hospitals takes place prior to surgery to prevent infection, they note.

(credit: CDC)
The paper is timed to coincide with the CDC’s “Get Smart About Antibiotics Week,” November 14–20, which aims to improve the antibiotic prescribing habits of clinicians to help combat the continuing rise of resistant strains of pathogenic bacteria.
“In addition to emerging resistance, the adverse events of decades of antibiotic use may have led to a number of other adverse consequences,” warns Surgical Infections Editor-in-Chief Donald E. Fry, MD, Northwestern University Feinberg School of Medicine, in an associated open-access editorial, “The Unintended Consequences of Antibiotic Use.”
(credit: CDC)
“The lesson of giving the preventive antibiotics before incision and subsequent contamination has been learned, but the lesson of discontinuation of the antibiotic after the incision has been closed continues to be problematic,” he notes.
“Prolonged preventive antibiotics after incision closure do not prevent infections, but they do change the composition of bacteria in the host at other anatomic locations. The result is resistant colonization of the patient and potentially Clostridium difficile infection. The aggregate effect of prolonged and unnecessary preventive antibiotics is more resistant pathogens in our hospitals.”
Risks of developing antibiotic resistance vs. prevention
A recent KurzweilAI news article reports that an international research team has developed a new antimicrobial peptide, clavanin-MO, that kills strains resistant to existing antibiotics. The researchers advised that the peptide could also be embedded in surfaces such as tabletops to make them resistant to microbial growth, as antimicrobial coatings for catheters, and in ointments to treat skin infections. Two KurzweilAI readers (“tedhowardnz” and “DevilDocNowCiv”) questioned this advice, suggesting that we need to minimize exposure to prevent antibiotic resistance.
One of the senior authors of the associated paper, César de la Fuente Nunez, has replied: “I agree exposure should be limited and regulated. Over-exposure and misuse of conventional antibiotics is one of the main reason bacteria have developed resistance to these drugs. However, it has recently been estimated that if we don’t develop novel therapies, drug-resistant infections will kill 10 million people annually, which corresponds to 1 death every 3 seconds. [We] need to come up with alternative strategies to treat these infections [that have] driven much of our research, including this study.
“Interestingly, work by others has shown that peptides, similar to the ones we have described here, do not readily select for resistance, particularly when compared to conventional antibiotics. We believe this is due to their multifunctional mechanism of action (i.e., they have multiple targets). We envision these peptides being used in adjuvant therapies with antibiotics, as they can potentiate antibiotic action, therefore reducing levels of both peptide and antibiotics required for effective treatment. This approach will help further reduce selective pressure towards the development of resistance.
“Hospital procedures currently sterilize surfaces using ethanol, etc. We think these peptides could be useful, for instance, to coat catheters to prevent microbial colonization. Catheters are often infected, meaning the patient has to undergo surgery to replace the catheter, with all the costs that are associated with this procedure. We think peptides may help prevent some of these infections.”
In related news …
- A multi-drug resistant mycobacteria lung infection (Mycobacterium abscessus) that can cause life-threatening illness in people with cystic fibrosis (CF) and can spread from patient to patient has now spread globally and is becoming increasingly virulent, according to new research published November 11 in the journal Science. The mystery: how does the pathogen manage to spread globally? The researchers speculate that healthy individuals may be unwittingly carrying the mycobacteria between countries, possibly via fomites (carriers, such as a dish or an article of clothing) and aerosols (particles dispersed in air or a gas).
- A previously unnoticed global outbreak of Mycobacterium chimaera, an invasive, slow-growing bacterium, is linked to heater-cooler devices (HCD) used in cardiac surgery, according to a study published Nov. 14 in Infection Control & Hospital Epidemiology. HCDs are stand-alone devices needed for heat exchange in heart-lung machines used in some 250,000 surgeries annually in the U.S., according to the CDC.
- Symptom-free Ebola infections have been found by researchers in 14 individuals in Sierra Leone, West Africa, who showed no signs of Ebola but have evidence of prior Ebola infection in their immune systems. (This open-access link to a PLOS Neglected Tropical Diseases paper goes live upon publication.)
* Coauthored by Senior author John E. Mazuski, MD; Massimo Sartelli, Macerata Hospital (Italy); and colleagues from John Peter Smith Health Network (Fort Worth, TX), Maggiore Hospital (Parma, Italy), Papa XXIII (Bergamo, Italy), University of Texas Health Science Center (Houston), UNIVPM (Ancona, Italy), Vanderbilt University Medical Center (Nashville, TN), and Washington University School of Medicine (St. Louis, MO). Mazuski has received research support from Astra-Zeneca, Bayer, and Merck, and honoraria as an advisory board member, consultant, or speaker from Allergan, Astra-Zeneca, Bayer, and Merck. Mazuski also receives grant support from the National Institutes of Health for collaborative work on an infection control research project. He serves as the President of the Surgical Infection Society without compensation. For the remaining authors, no competing financial interests exist.
Abstract of Antimicrobial Stewardship: A Call to Action for Surgeons