Piggy-backing proteins ride white blood cells to destroy metastasizing cancer
January 8, 2014
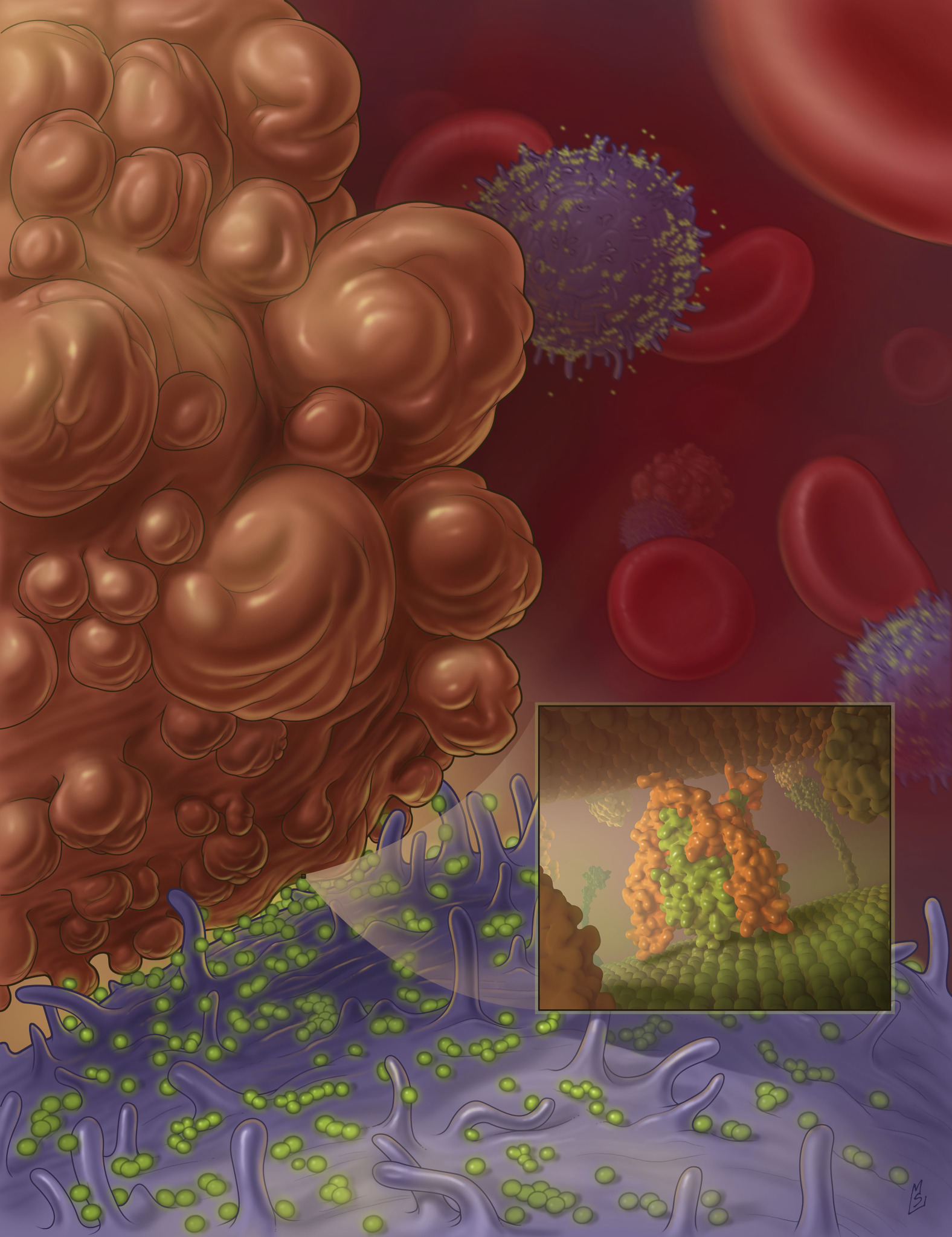
Nanoscale liposomes with TRAIL protein (in green) attach to the surface of white blood cells, and are available in the blood flow to bump into cancer cells (brown) and program them to die (credit: Cornell University)
Cornell biomedical engineers have discovered a new way to destroy metastasizing cancer cells traveling through the bloodstream by hitching cancer-killing proteins along for a ride on life-saving white blood cells.
“These circulating cancer cells are doomed,” said Michael King, Cornell professor of biomedical engineering and the study’s senior author.
“About 90 percent of cancer deaths are related to metastases, but now we’ve found a way to dispatch an army of killer white blood cells that cause apoptosis — the cancer cell’s own death — obliterating them from the bloodstream.
“When surrounded by these guys, it becomes nearly impossible for the cancer cell to escape.”
Metastasis is the spread of a cancer cells to other parts of the body. Surgery and radiation are effective at treating primary tumors, but difficulty in detecting metastatic cancer cells has made treatment of the spreading cancers problematic, say the scientists.
King and his colleagues injected human blood samples, and later mice, with two proteins: E-selectin (ES, an adhesive) and TRAIL (Tumor Necrosis Factor Related Apoptosis-Inducing Ligand).
The TRAIL protein joined with the E-selectin protein was able to stick to leukocytes (white blood cells) abundant in the bloodstream. When a cancer cell comes into contact with TRAIL, which is nearly unavoidable in the frenzied flow of blood, the cancer cell essentially kills itself.
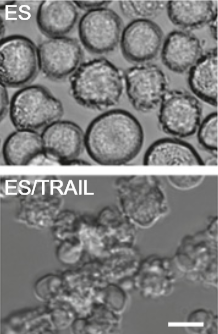
Colorectal adenocarcinoma cancer cell morphology after treatment with ES (adhesive) and with added TRAIL (killer proteins) (scale bar, 20 microns) (credit: Michael J. Mitchell et al./PNAS)
Nearly 100 percent effectiveness in lab test
Using white blood cells in flowing blood is “more effective than directly targeting the cancer cells with liposomes or soluble protein,” say the authors.
When treating cancer cells with the proteins in saline, King and his colleagues found a 60 percent success rate in killing the cancer cells.
In normal laboratory conditions, the saline lacks white blood cells to serve as a carrier for the adhesive and killer proteins.
Once the proteins were added to flowing blood that mimicked human-body conditions, however, the success rate in killing the cancer cells jumped to nearly 100 percent.
The National Cancer Institute of the National Institutes of Health funded the research through Cornell’s Center for the Microenvironment and Metastasis.
Abstract of Proceedings of the National Academy of Sciences paper
Metastasis through the bloodstream contributes to poor prognosis in many types of cancer. Mounting evidence implicates selectin-based adhesive interactions between cancer cells and the blood vessel wall as facilitating this process, in a manner similar to leukocyte trafficking during inflammation. Here, we describe a unique approach to target and kill colon and prostate cancer cells in the blood that causes circulating leukocytes to present the cancer-specific TNF-related apoptosis inducing ligand (TRAIL) on their surface along with E-selectin adhesion receptor. This approach, demonstrated in vitro with human blood and also in mice, mimics the cytotoxic activity of natural killer cells and increases the surface area available for delivery of the receptor-mediated signal. The resulting “unnatural killer cells” hold promise as an effective means to neutralize circulating tumor cells that enter blood with the potential to form new metastases.