Protons found to pass through graphene, raising hopes for efficient fuel cells
December 1, 2014
Graphene, an atomic-scale honeycomb lattice made of carbon atoms, allows protons to freely pass through it, contrary to previous thinking (credit: Wikimedia Commons)
Graphene, which is impermeable to all gases and liquids, can actually allow protons to pass through it, University of Manchester researchers have found, to their surprise.
Published in the journal Nature, the discovery could revolutionize fuel cells and other hydrogen-based technologies, the researchers say, because that’s exactly what fuel cells require: a barrier that only allows protons (hydrogen atoms with their electrons stripped off) to pass through, while blocking hydrogen.
In addition, graphene membranes could be used to extract hydrogen gas out of the atmosphere, where it is present in minute quantities — creating the possibility of electric generators powered by air, the researchers say.
One-atom thick material graphene, first isolated and explored in 2004 by a team at the University of Manchester, is renowned for its barrier properties, which has a number of uses in applications such as corrosion-proof coatings and impermeable packaging. For example, it would take billions of years for hydrogen, the smallest of all atoms, to pierce a graphene monolayer.
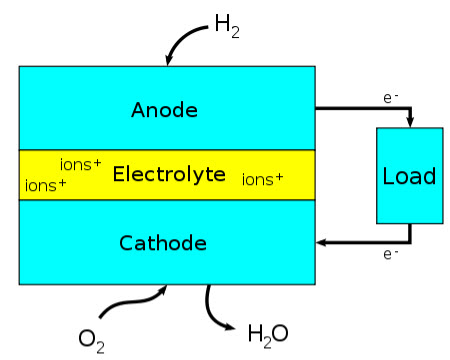
How a fuel cell works (credit: Wikimedia Commons)
The discovery was made by a group led by Sir Andre Geim, who was awarded the 2010 Nobel Prize in Physics jointly with Konstantin Novoselov for his work on graphene.
The discovery makes monolayers of graphene (and also its sister material boron nitride) attractive for possible uses as proton-conducting membranes, which are at the heart of modern fuel cell technology, especially at elevated temperatures and if the films are covered with catalytic nanoparticles such as platinum.
Fuel cells use oxygen and hydrogen as a fuel and convert the input chemical energy directly into electricity. Without membranes that allow an exclusive flow of protons but prevent other species to pass through, this technology would not exist.
Despite being well-established, fuel-cell technology requires further improvements to make it more widely used. One of the major problems is a fuel crossover through the existing proton membranes, which reduces their efficiency and durability.
The University of Manchester research suggests that the use of graphene or monolayer boron nitride can allow the existing membranes to become thinner and more efficient, with less fuel crossover and poisoning. This can boost competitiveness of fuel cells.
The Manchester group also demonstrated that their one-atom-thick membranes can be used to extract hydrogen from a humid atmosphere. They hypothesize that such harvesting can be combined with fuel cells to create a mobile electric generator fueled simply by the hydrogen present in air.
Marcelo Lozada-Hidalgo, a PhD student and corresponding author of this paper, said: “When you know how it should work, it is a very simple setup. You put a hydrogen-containing gas on one side, apply small electric current and collect pure hydrogen on the other side. This hydrogen can then be burned in a fuel cell.
“We worked with small membranes, and the achieved flow of hydrogen is of course tiny so far. But this is the initial stage of discovery, and the paper is to make experts aware of the existing prospects. To build up and test hydrogen harvesters will require much further effort.”
The work is an international collaboration involving groups from China and the Netherlands who supported theoretical aspects of this research. Marcelo Lozada-Hidalgo is funded by a PhD studentship program between the National Council of Science and Technology of Mexico and The University of Manchester.
Abstract of Proton transport through one-atom-thick crystals
Graphene is increasingly explored as a possible platform for developing novel separation technologies1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. This interest has arisen because it is a maximally thin membrane that, once perforated with atomic accuracy, may allow ultrafast and highly selective sieving of gases, liquids, dissolved ions and other species of interest2, 9, 10, 11, 12,13, 14, 15, 16, 17, 18, 19. However, a perfect graphene monolayer is impermeable to all atoms and molecules under ambient conditions1, 2, 3, 4, 5, 6, 7: even hydrogen, the smallest of atoms, is expected to take billions of years to penetrate graphene’s dense electronic cloud3, 4, 5, 6. Only accelerated atoms possess the kinetic energy required to do this20, 21. The same behaviour might reasonably be expected in the case of other atomically thin crystals22, 23. Here we report transport and mass spectroscopy measurements which establish that monolayers of graphene and hexagonal boron nitride (hBN) are highly permeable to thermal protons under ambient conditions, whereas no proton transport is detected for thicker crystals such as monolayer molybdenum disulphide, bilayer graphene or multilayer hBN. Protons present an intermediate case between electrons (which can tunnel easily through atomically thin barriers24) and atoms, yet our measured transport rates are unexpectedly high4, 5 and raise fundamental questions about the details of the transport process. We see the highest room-temperature proton conductivity with monolayer hBN, for which we measure a resistivity to proton flow of about 10 Ω cm2 and a low activation energy of about 0.3 electronvolts. At higher temperatures, hBN is outperformed by graphene, the resistivity of which is estimated to fall below 10−3 Ω cm2 above 250 degrees Celsius. Proton transport can be further enhanced by decorating the graphene and hBN membranes with catalytic metal nanoparticles. The high, selective proton conductivity and stability make one-atom-thick crystals promising candidates for use in many hydrogen-based technologies.