Quadriplegic man is first to regain use of hand and fingers
April 15, 2016

Ian Burkhart, who is paralyzed, playing a guitar video game, made possible by a new neural bypass system. (credit: The Ohio State Wexner Medical Center and Battelle)
Battelle and The Ohio State University Wexner Medical Center have developed and surgically implanted the first neural bypass for spinal cord injuries that reconnects the brain to muscles, allowing voluntary and functional control of a paralyzed limb by using a patient’s thoughts — no robotic prosthetic arm required.
Ian Burkhart, a 24-year-old quadriplegic injured in a diving accident, is the first person to experience “limb reanimation,” thanks to this neural bypass system, called “NeuroLife.”
The Battelle team has been developing this technology for more than a decade. Battelle scientists first recorded neural impulses from an electrode array* implanted in a paralyzed person’s brain. They used that recorded data to illustrate and test the device’s effect on the patient and prove the concept.
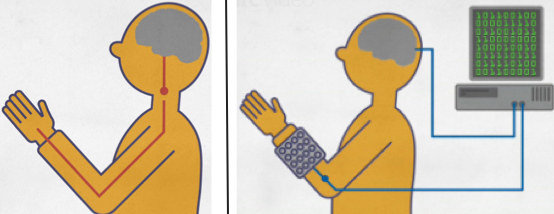
How the neural bypass system works. Left: Injury to spinal cord at C7 blocks signals from the motor cortex from reaching the arm. Right: 96 implanted microelectrodes pick up motor-cortex signals and send to a computer, whose algorithms learn and decode hand and finger movement thoughts, converting them to electrical signals that then activate an electrical stimulation sleeve, which stimulates matching neurons in the patient’s wrist to trigger hand and finger movements. (credit: The Ohio State Wexner Medical Center and Battelle)
The Ohio State and Battelle teams worked together to figure out the correct sequence of electrodes to stimulate to allow Burkhart to move his fingers and hand functionally. Burkhart can now perform sophisticated movements with his hands and fingers, such as picking up a spoon, swiping a credit card, or picking up and holding a phone to his ear. For example, he uses different brain signals and muscles to rotate his hand, make a fist, or pinch his fingers together to grasp an object. (He retained some functionality in his right shoulder and bicep.)

Burkhart swiping a credit card (credit: The Ohio State Wexner Medical Center and Battelle)
“It’s amazing to see what he’s accomplished,” said Nick Annetta, electrical engineering lead for Battelle’s team on the project. “Ian can grasp a bottle, pour the contents of the bottle into a jar and put the bottle back down. Then he takes a stir bar, grips that and then stirs the contents of the jar that he just poured and puts it back down. He’s controlling it every step of the way.”
The neural bypass technology combines computer algorithms that learn and decode the user’s brain activity and a high-definition muscle stimulation sleeve that triggers neurons in the hand and finger.
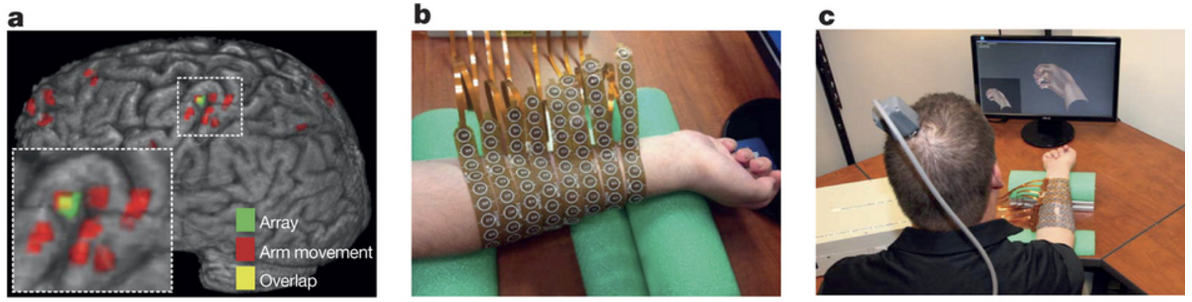
Neural bypass system experimental setup and neural modulation. (a) Red regions are brain areas most active during attempts to mimic hand movements, as indicated by fMRI (functional magnetic imaging resonance) contrast. The implanted microelectrode array location from post-operation computed tomography is shown in green; The overlap of the red and green regions is shown in yellow. (b) Neuromuscular electrical stimulation sleeve. (c) Neural bypass system learning to match patient’s thoughts (about hand activity shown on computer monitor) to specific activation patterns in the electrical stimulation sleeve. (credit: Chad E. Bouton et al./Nature)
“In the 30 years I’ve been in this field, this is the first time we’ve been able to offer realistic hope to people who have very challenging lives,” said Jerry Mysiw, M.D., chair of the Department of Physical Medicine and Rehabilitation at Ohio State. “What we’re looking to do is help these people regain more control over their bodies.”
Burkhart is the first of a potential five participants in a clinical study. The researchers say this technology promises to help patients affected by various brain and spinal cord injuries such as strokes and traumatic brain injury to be more independent and functional.
Regrettably, Burkhart will not be able to take the system home when funding runs out this year, but the researchers hope to evolve the technology into a wireless system and make it readily available to be used by patients at home.
The research was published online in the journal Nature on April 13.
OSU Wexner Medical Center | Man Uses His Own Brainwaves To Retrain His Paralyzed Hand
Nature Video | The nerve bypass: how to move a paralysed hand
* Widely reported as a “computer chip” (based on an inaccurate statement in the Ohio State press release).
Abstract of Restoring cortical control of functional movement in a human with quadriplegia
Millions of people worldwide suffer from diseases that lead to paralysis through disruption of signal pathways between the brain and the muscles. Neuroprosthetic devices are designed to restore lost function and could be used to form an electronic ‘neural bypass’ to circumvent disconnected pathways in the nervous system. It has previously been shown that intracortically recorded signals can be decoded to extract information related to motion, allowing non-human primates and paralysed humans to control computers and robotic arms through imagined movements. In non-human primates, these types of signal have also been used to drive activation of chemically paralysed arm muscles. Here we show that intracortically recorded signals can be linked in real-time to muscle activation to restore movement in a paralysed human. We used a chronically implanted intracortical microelectrode array to record multiunit activity from the motor cortex in a study participant with quadriplegia from cervical spinal cord injury. We applied machine-learning algorithms to decode the neuronal activity and control activation of the participant’s forearm muscles through a custom-built high-resolution neuromuscular electrical stimulation system. The system provided isolated finger movements and the participant achieved continuous cortical control of six different wrist and hand motions. Furthermore, he was able to use the system to complete functional tasks relevant to daily living. Clinical assessment showed that, when using the system, his motor impairment improved from the fifth to the sixth cervical (C5–C6) to the seventh cervical to first thoracic (C7–T1) level unilaterally, conferring on him the critical abilities to grasp, manipulate, and release objects. This is the first demonstration to our knowledge of successful control of muscle activation using intracortically recorded signals in a paralysed human. These results have significant implications in advancing neuroprosthetic technology for people worldwide living with the effects of paralysis.