Rapidly building artificial arteries for testing drugs
February 26, 2016
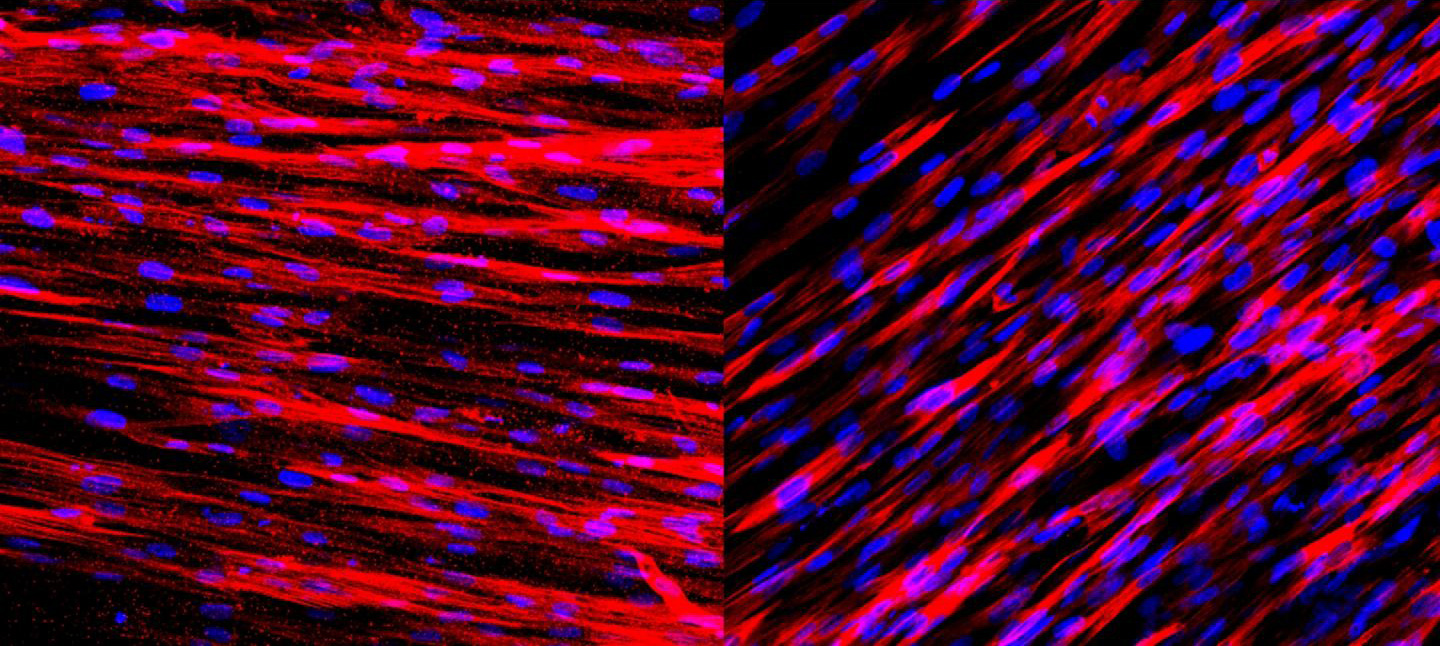
The muscle layer of the engineered human arteries function well. After just one week, the arteries contain two types of proteins important for muscle contraction — actin (stained red, left) and calponin (stained red, right). These two protein molecules allow the arteries to contract and dilate in response to environmental stimuli. (credit: George Truskey Lab, Duke University)
Duke University researchers have developed a rapid new technique for making small-scale artificial human arteries for use in a system for testing drugs — one that is more accurate and reliable than using animal models. That means promising drugs could be better tested before entering human trials.
The new technique produces the artificial arteries ten times faster than current methods and the arteries are functional.
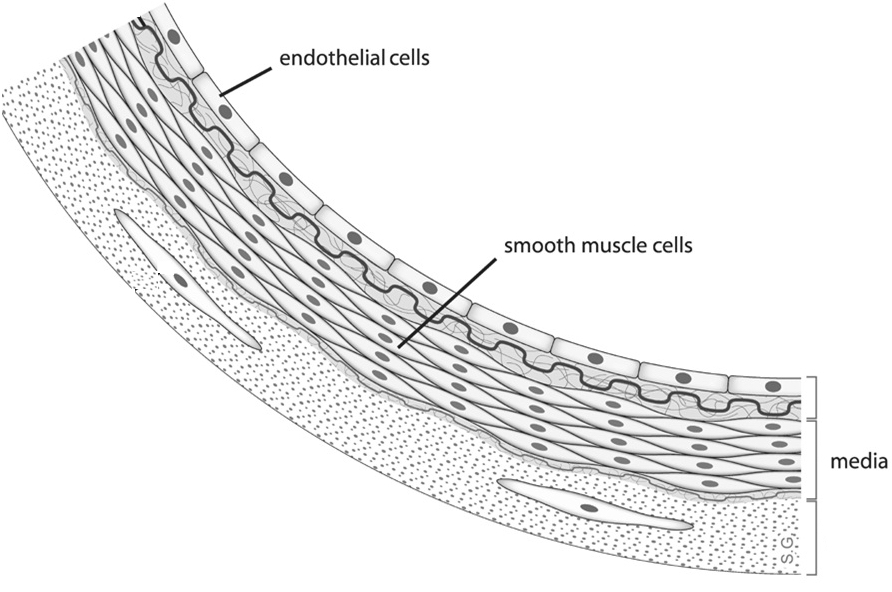
Arterial blood vessel walls have multiple layers of cells, including the endothelium and media. The endothelium is the innermost lining that interacts with circulating blood. The media is made mostly of smooth muscle cells that help control the flow of blood and blood pressure. These two layers communicate and control how blood vessels react to stimuli such as drugs and exercise. (credit: Wikimedia Commons)
The researchers successfully engineered artificial arteries containing the lining (endothelium) and muscle (media) layers of arteries. They also showed that both layers could communicate and function normally.
“We wanted to focus on arteries because that’s where most of the damage is caused in coronary diseases,” said George Truskey, senior author, Professor of Biomedical Engineering and Dean of the Pratt School of Engineering at Duke University.
How to create an artificial artery
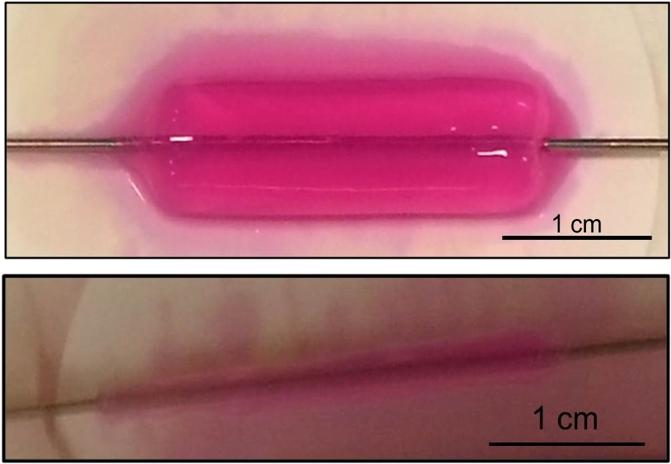
To rapidly construct strong human artificial arteries, cells were embedded in collagen gels (top) and then more than 90 per cent of the water was removed (bottom). These arteries were prepared in only three hours. The mechanical strength of these engineered arteries means researchers no longer need to wait six to eight weeks for the tissues to mature. (credit: George Truskey Lab, Duke University)
Graduate student Cristina Fernandez developed the technique to create arteries; but instead of full-size arteries, they were scaled down to one-tenth the size of a typical human artery. With this smaller diameter, the researchers were able to make a lot of these artificial vessels in a short amount of time and use them in experiments in just a few hours, instead of spending several weeks developing each one.
Despite the smaller size, these engineered arteries behaved normally, with statin drugs blocking inflammation just as they do in patients. The endothelial cells also released chemical signals to relax and constrict the media layer, again just like they do in the normal human body.
“Many of the [previous] techniques for creating artificial tissue also were rather lengthy, which was frustrating,” said Truskey. The previous studies also focused on the media cells rather than the endothelial cells, and “nobody had shown how the two would interact,” he added.
Replacing arteries in patients
“While our arteries are small and intended for testing, they’re just as mechanically strong as those intended to be put inside of the body,” said Truskey. “So the technique could be beneficial to researchers trying to create artificial arteries to replace damaged ones in patients as well.” The arteries could also be used to look at how some select rare genetic diseases affect arteries, he added.
The study was reported online (open access) in Nature Scientific Reports. The work was supported by the National Institutes of Health and the American Heart Association.
Abstract of Human Vascular Microphysiological System for in vitro Drug Screening
In vitro human tissue engineered human blood vessels (TEBV) that exhibit vasoactivity can be used to test human toxicity of pharmaceutical drug candidates prior to pre-clinical animal studies. TEBVs with 400–800 μM diameters were made by embedding human neonatal dermal fibroblasts or human bone marrow-derived mesenchymal stem cells in dense collagen gel. TEBVs were mechanically strong enough to allow endothelialization and perfusion at physiological shear stresses within 3 hours after fabrication. After 1 week of perfusion, TEBVs exhibited endothelial release of nitric oxide, phenylephrine-induced vasoconstriction, and acetylcholine-induced vasodilation, all of which were maintained up to 5 weeks in culture. Vasodilation was blocked with the addition of the nitric oxide synthase inhibitor L-NG-Nitroarginine methyl ester (L-NAME). TEBVs elicited reversible activation to acute inflammatory stimulation by TNF-α which had a transient effect upon acetylcholine-induced relaxation, and exhibited dose-dependent vasodilation in response to caffeine and theophylline. Treatment of TEBVs with 1 μM lovastatin for three days prior to addition of Tumor necrosis factor – α (TNF-α) blocked the injury response and maintained vasodilation. These results indicate the potential to develop a rapidly-producible, endothelialized TEBV for microphysiological systems capable of producing physiological responses to both pharmaceutical and immunological stimuli.