Rechargeable batteries with almost infinite lifetimes coming, say MIT-Samsung engineers
August 24, 2015
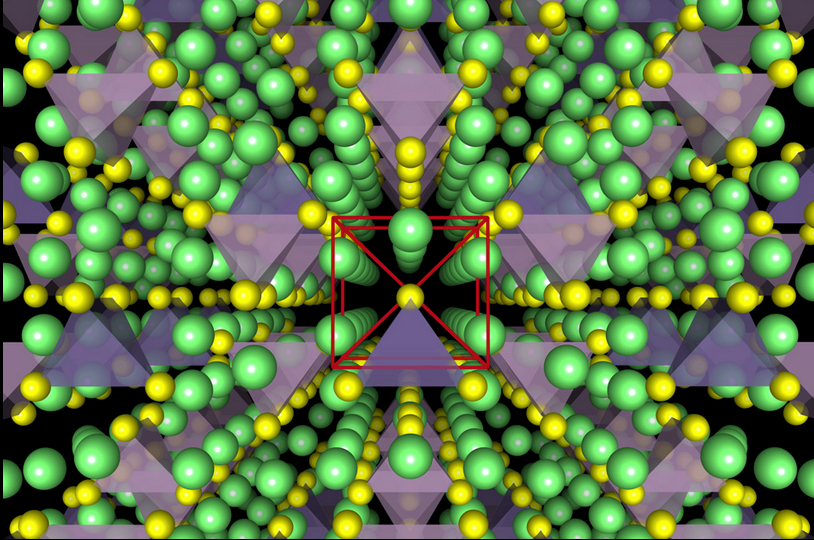
Illustration of the crystal structure of a superionic conductor. The backbone of the material is a cubic-like arrangement of sulphur anions (yellow). Lithium atoms are depicted in green, PS4 tetrahedra in violet, and GeS4 tetrahedra in blue. (credit: Yan Wang)
MIT and Samsung researchers have developed a new approach to achieving long life and a 20 to 30 percent improvement in power density (the amount of power stored in a given space) in rechargeable batteries — using a solid electrolyte, rather than the liquid used in today’s most common rechargeables. The new materials could also greatly improve safety and last through “hundreds of thousands of cycles.”
The results are reported in the journal Nature Materials. Solid-state electrolytes could be “a real game-changer,” says co-author Gerbrand Ceder, MIT visiting professor of materials science and engineering, creating “almost a perfect battery, solving most of the remaining issues” in battery lifetime, safety, and cost.
Superionic lithium-ion conductors
The electrolyte in rechargeable batteries is typically a liquid organic solvent whose function is to transport charged particles from one of a battery’s two electrodes to the other during charging and discharging. That material has been responsible for the overheating and fires that, for example, resulted in a temporary grounding of all of Boeing’s 787 Dreamliner jets.
With a solid electrolyte, there’s no safety problem, he says. “You could throw it against the wall, drive a nail through it — there’s nothing there to burn.”
The key to making all this feasible, Ceder says, was finding solid materials that could conduct ions fast enough to be useful in a battery. The initial findings focused on a class of materials known as superionic lithium-ion conductors, which are compounds of lithium, germanium, phosphorus, and sulfur. But the principles derived from this research could lead to even more effective materials, the team says, and they could function below about minus 20 degrees Fahrenheit.
Researchers at the University of California at San Diego and the University of Maryland were also involved in the study.
The article title was corrected to read “infinite” instead of indefinite.
Abstract of Design principles for solid-state lithium superionic conductors
Lithium solid electrolytes can potentially address two key limitations of the organic electrolytes used in today’s lithium-ion batteries, namely, their flammability and limited electrochemical stability. However, achieving a Li+ conductivity in the solid state comparable to existing liquid electrolytes (>1 mS cm−1) is particularly challenging. In this work, we reveal a fundamental relationship between anion packing and ionic transport in fast Li-conducting materials and expose the desirable structural attributes of good Li-ion conductors. We find that an underlying body-centred cubic-like anion framework, which allows direct Li hops between adjacent tetrahedral sites, is most desirable for achieving high ionic conductivity, and that indeed this anion arrangement is present in several known fast Li-conducting materials and other fast ion conductors. These findings provide important insight towards the understanding of ionic transport in Li-ion conductors and serve as design principles for future discovery and design of improved electrolytes for Li-ion batteries.