Researchers hack cell biology to create complex shapes that form living tissue
January 5, 2018
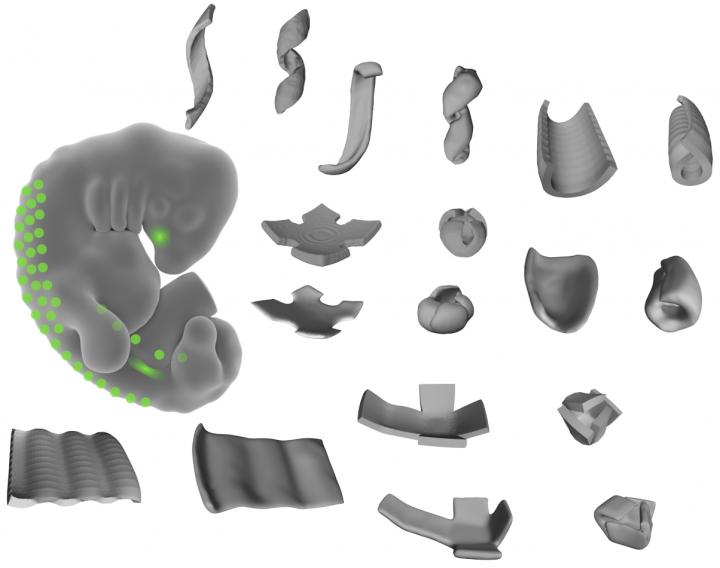
This image shows the shapes made of living tissue, engineered by the researchers. By patterning mechanically active mouse or human cells to thin layers of extracellular fibers, the researchers could create bowls, coils, and ripple shapes. (credit: Alex Hughes)
Many of the complex folded and curved shapes that form human tissues can now be programmatically recreated with very simple instructions, UC San Francisco (UCSF) bioengineers report December 28 in the journal Developmental Cell.
The researchers used 3D cell-patterning to shape active mouse and human embryonic cells into thin layers of extracellular matrix fibers (a structural material produced by human cells that make up our connective tissue) to create bowls, coils, and ripples out of living tissue. A web of these fibers folded themselves up in predictable ways, mimicking developmental processes in natural human body tissue.
Beyond 3D-printing and molds
As KurzweilAI has reported, labs have already used modified 3D printers to pioneer 3D shapes for tissue engineering (such as this research in creating an ear and jawbone structure). They have also used micro-molding for creating variously shaped objects using plastic material in a mold (frame). But the final product often misses key structural features of normal tissues.
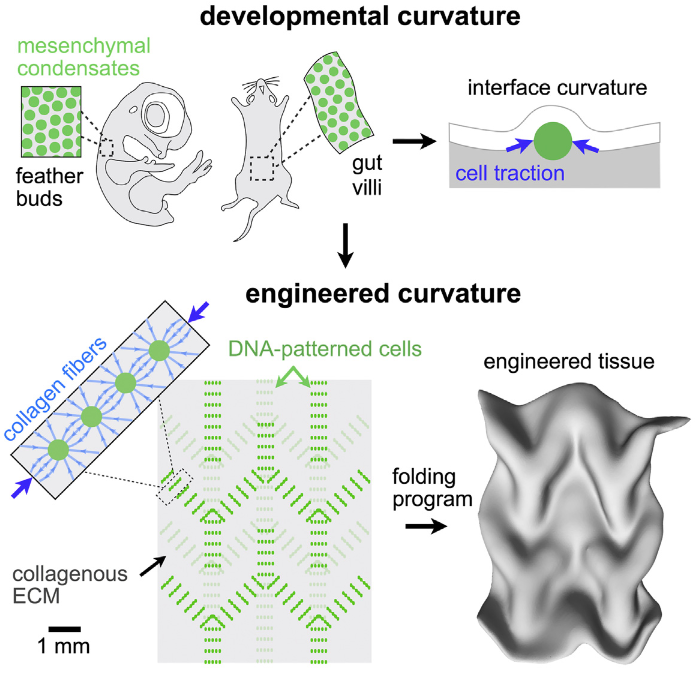
Engineered tissue curvature using DNA-programmed assembly of cells (credit: Alex J. Hughes et al./ Developmental Cell)
The UCSF lab approach instead used a precision 3D cell-patterning technology called DNA-programmed assembly of cells (DPAC). It provides an initial template (pattern) for tissue to later develop in vitro (in a test tube or other lab container). That tissue automatically folds itself into complex shapes in ways that replicate how in vivo (body) tissues normally assemble themselves hierarchically during development.
“This approach could significantly improve the structure, maturation, and vascularization” of tissues in organoids” (miniature models of human parts, such as brains, used for drug testing) “and 3D-printed tissues in general,” the researchers note in the paper.
“We believe these efforts have important implications for the engineering of in vitro models of disease, for regenerative medicine, and for future applications of living active materials such as in soft robotics. … These mechanisms can be integrated with top-down patterning technologies such as optogenetics, micromolding, and printing approaches that control cellular and [extracellular matrix] tissue composition at specific locations.”
This work was funded by a Jane Coffin Childs postdoctoral fellowship, the National Institutes of Health, the Department of Defense Breast Cancer Research Program, the NIH Common Fund, the Chan-Zuckerberg Biohub Investigator Program, the National Science Foundation, the UCSF Program in Breakthrough Biomedical Research, and the UCSF Center for Cellular Construction.
Abstract of Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme
Many tissues fold into complex shapes during development. Controlling this process in vitro would represent an important advance for tissue engineering. We use embryonic tissue explants, finite element modeling, and 3D cell-patterning techniques to show that mechanical compaction of the extracellular matrix during mesenchymal condensation is sufficient to drive tissue folding along programmed trajectories. The process requires cell contractility, generates strains at tissue interfaces, and causes patterns of collagen alignment around and between condensates. Aligned collagen fibers support elevated tensions that promote the folding of interfaces along paths that can be predicted by modeling. We demonstrate the robustness and versatility of this strategy for sculpting tissue interfaces by directing the morphogenesis of a variety of folded tissue forms from patterns of mesenchymal condensates. These studies provide insight into the active mechanical properties of the embryonic mesenchyme and establish engineering strategies for more robustly directing tissue morphogenesis ex vivo.