Robotic arm precisely controlled by thought
May 22, 2015
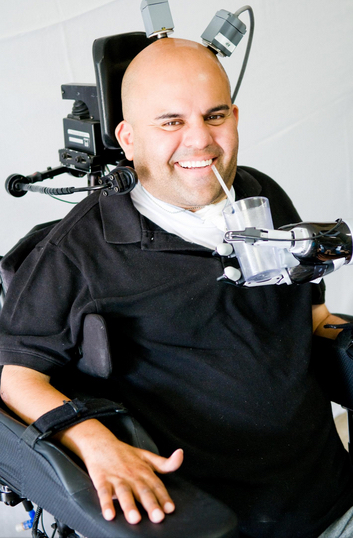
Erik Sorto smoothly controls robotic arm with his brain (credit: Spencer Kellis and Christian Klaes /Caltech)
Paralyzed from the neck down, Erik G. Sorto now can smoothly move a robotic arm just by thinking about it, thanks to a clinical collaboration between Caltech, Keck Medicine of USC and Rancho Los Amigos National Rehabilitation Center,
Previous neural prosthetic devices, such as Braingate, were implanted in the motor cortex, resulting in delayed, jerky movements. The new device was implanted in the posterior parietal cortex (PPC), a part of the brain that controls the intent to move, not the movement directly.
That makes Sorto, who has been paralyzed for over 10 years, the first quadriplegic person in the world to perform a fluid hand-shaking gesture or play “rock, paper, scissors,” using a robotic arm.
In April 2013, Keck Medicine of USC surgeons implanted a pair of small electrode arrays in two parts of the posterior parietal cortex, one that controls reach and another that controls grasp.
Each 4-by-4 millimeter array contains 96 active electrodes that, in turn, each record the activity of single neurons in the PPC. The arrays are connected by a cable to a system of computers that process the signals, to decode the brain’s intent and control output devices, such as a computer cursor and a robotic arm.
Although he was able to immediately move the robot arm with his thoughts, after weeks of imagining, Sorto refined his control of the arm.
Now, Sorto is able to execute advanced tasks with his mind, such as controlling a computer cursor; drinking a beverage; making a hand-shaking gesture; and performing various tasks with the robotic arm.
Designed to test the safety and effectiveness of this new approach, the clinical trial was led by principal investigator Richard Andersen, the James G. Boswell Professor of Neuroscience at Caltech, neurosurgeon Charles Y. Liu, professor of neurological surgery and neurology at the Keck School of Medicine of USC and biomedical engineering at USC, and neurologist Mindy Aisen, chief medical officer at Rancho Los Amigos.
Aisen, also a clinical professor of neurology at the Keck School of Medicine of USC, says that advancements in prosthetics like these hold promise for the future of patient rehabilitation.

NeuroPort microelectrode array implanted in Erik Sorto’s posterior parietal cortex (credit: Blackrock Microsystems)
“This research is relevant to the role of robotics and brain-machine interfaces as assistive devices, but also speaks to the ability of the brain to learn to function in new ways,” Aisen said. “We have created a unique environment that can seamlessly bring together rehabilitation, medicine, and science as exemplified in this study.”
Sorto has signed on to continue working on the project for a third year. He says the study has inspired him to continue his education and pursue a master’s degree in social work.
The results of the clinical trial appear in the May 22, 2015, edition of the journal Science. The implanted device and signal processors used in the clinical trial were the NeuroPort Array and NeuroPort Bio-potential Signal Processors developed by Blackrock Microsystems in Salt Lake City, Utah. The robotic arm used in the trial was the Modular Prosthetic Limb, developed at the Applied Physics Laboratory at Johns Hopkins.
This trial was funded by the National Institutes of Health, the Boswell Foundation, the Department of Defense, and the USC Neurorestoration Center.
Caltech | Next Generation of Neuroprosthetics: Science Explained — R. Andersen May 2015
Keck Medicine of USC | Next Generation of Neuroprosthetics: Erik’s Story
Abstract of Decoding motor imagery from the posterior parietal cortex of a tetraplegic human
Nonhuman primate and human studies have suggested that populations of neurons in the posterior parietal cortex (PPC) may represent high-level aspects of action planning that can be used to control external devices as part of a brain-machine interface. However, there is no direct neuron-recording evidence that human PPC is involved in action planning, and the suitability of these signals for neuroprosthetic control has not been tested. We recorded neural population activity with arrays of microelectrodes implanted in the PPC of a tetraplegic subject. Motor imagery could be decoded from these neural populations, including imagined goals, trajectories, and types of movement. These findings indicate that the PPC of humans represents high-level, cognitive aspects of action and that the PPC can be a rich source for cognitive control signals for neural prosthetics that assist paralyzed patients.