Robust ‘spider silk’ matrix guides cardiac tissue regeneration
April 13, 2015
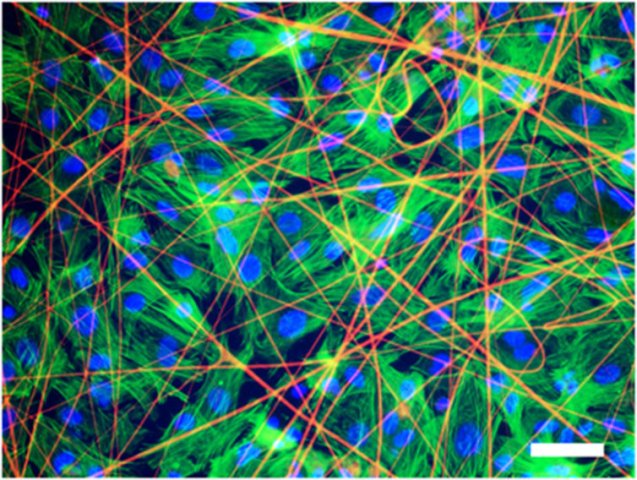
Heart tissue cells grown on a matrix with artificial spider-web protein, stained with fluorescent markers (credit: Alexander Teplenin et al./PLOS ONE)
Genetically engineered fibers of the protein spidroin — the construction material for spider webs — are a ideal matrix (substrate or frame) for cultivating heart tissue cells, Moscow Institute of Physics and Technology (MIPT) researchers have found, as noted in an open-access article in the journal PLOS ONE.
Regenerative methods can solve the problem of transplant rejection, but it’s a challenge to find a suitable matrix to grow cells on: The material should be non-toxic, elastic, and not rejected by the body or impede cell growth. KurzweilAI has reported on a number of solutions.
Researchers led by Professor Konstantin Agladze, who heads the Laboratory of the Biophysics of Excitable Systems at MIPT, have been cultivating tissues that contract and conduct excitation waves, from cells called cardiomyocytes.
They decided to explore using synthetic electrospun fibers of spidroin as a matrix. They’re light, five times stronger than steel, twice as elastic as nylon, and are capable of stretching a third of their length. Which is why they are currently used as a substrate to grow implants like bones, tendons and cartilages, as well as dressings.
But could they also function as a matrix for soft tissues, such as the heart?
Agladze decided to find out. His team seeded isolated neonatal rat cardiomyocytes on fiber matrices. Using a microscope and fluorescent markers, the researchers monitored the growth of the cells and tested their contractibility and the ability to conduct electric impulses, which are the main features of normal cardiac tissue.
Within three to five days a layer of cells formed on the substrate. They were able to contract synchronously and conduct electrical impulses just like the tissue of a living heart would.
“Cardiac tissue cells successfully adhere to the substrate of recombinant spidroin,” Agladze says. “They grow forming layers and are fully functional, which means they can contract coordinately.”
Abstract of Functional Analysis of the Engineered Cardiac Tissue Grown on Recombinant Spidroin Fiber Meshes
In the present study, we examined the ability of the recombinant spidroin to serve as a substrate for the cardiac tissue engineering. For this purpose, isolated neonatal rat cardiomyocytes were seeded on the electrospun spidroin fiber matrices and cultured to form the confluent cardiac monolayers. Besides the adhesion assay and immunostaining analysis, we tested the ability of the cultured cardiomyocytes to form a functional cardiac syncytium by studying excitation propagation in the cultured tissue with the aid of optical mapping. It was demonstrated that recombinant spidroin fiber meshes are directly suitable for the adherence and growth of the cardiomyocytes without additional coating with the attachment factors, such as fibronectin.