Scientists time-reverse developed stem cells to make them ’embryonic’ again
March 24, 2016
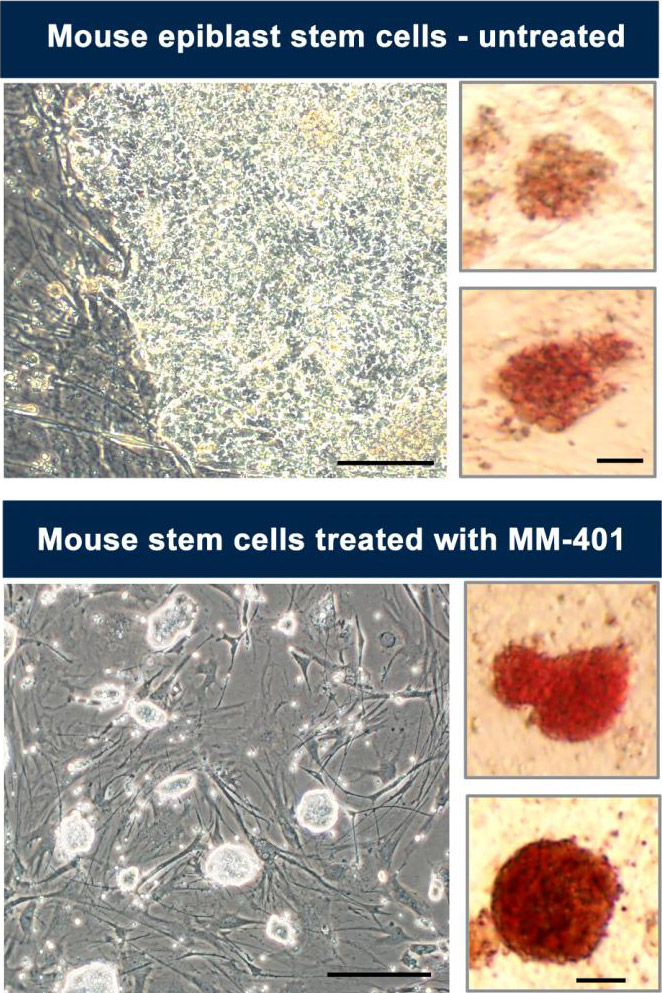
Researchers have reversed “primed” (developed) “epiblast” stem cells (top) from early mouse embryos using the drug MM-401, causing the treated cells (bottom) to revert to the original form of the stem cells. (credit: University of Michigan)
University of Michigan Medical School researchers have discovered a way to convert mouse stem cells (taken from an embryo) that have become “primed” (reached the stage where they can differentiate, or develop into every specialized cell in the body) to a “naïve” (unspecialized) state by simply adding a drug.
This breakthrough has the potential to one day allow researchers to avoid the ethically controversial use of human embryos left over from infertility treatments. To achieve this breakthrough, the researchers treated the primed embryonic stem cells (“EpiSC”) with a drug called MM-401* (a leukemia drug) for a short period of time.
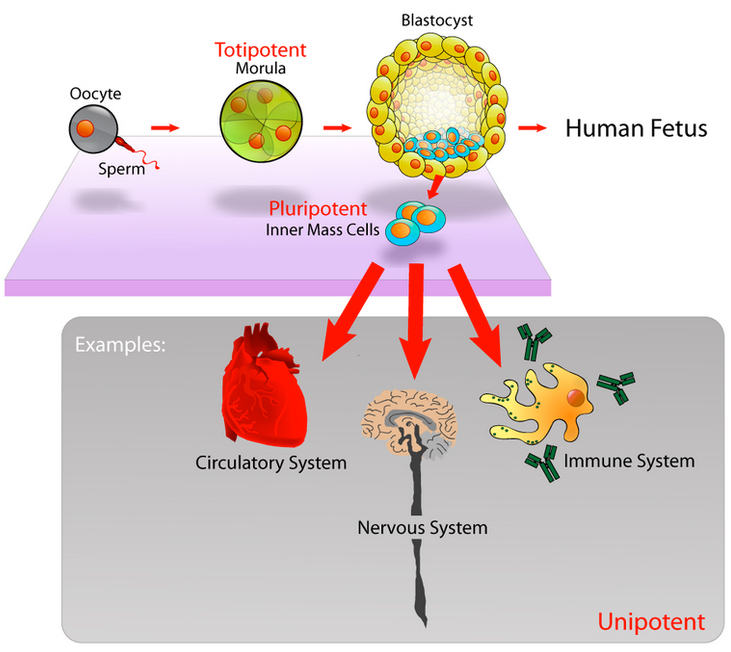
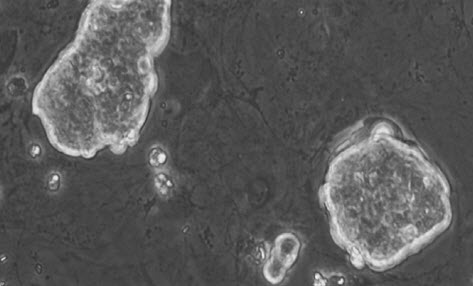
Embryonic stem cells are able to develop into any type of cell, except those of the placenta (credit: Mike Jones/CC)
Reverting back to the embryonic state
As the research team reports in the journal Cell Stem Cell, this drug treatment caused more than half of these EpiSC cells to return to a naïve (less specialized) state as “reverted embryonic stem cells” (rESC). The researchers then bred healthy mice from those reverted rESC cells — proving that the drug-treated cells were still viable and had the ability to become any type of cell (achieved pluripotency).
This study is significant because it’s the first time scientists have been able to make stem cells revert to their original state without complications. Also, the drug leaves no trace behind, whereas genetic modification of the stem cells (used by other researchers) may block the stem cells from developing into healthy cells. The researchers only needed to treat the EpiSCs with a single drug and for just a few days.
Past attempts by other teams to return mouse EpiSC cells to the original naïve state have either resulted in a far lower proportion of cells returned to a reverted state, or have produced cells that were not viable. Those past studies also needed to use cocktails of multiple drugs given over the long term.
The work was funded by the National Institutes of Health and the Leukemia and Lymphoma Society.
Human stem cells next
Meanwhile, scientists at the University of Cambridge have also just reported (in an open-access paper in the journal Stem Cell Reports) that they have also produced stem cells directly from embryos — but they did it with human stem cells (for the first time).
“Until now it hasn’t been possible to isolate these naïve stem cells, even though we’ve had the technology to do it in mice for thirty years — leading some people to doubt it would be possible,” says Ge Guo, the study’s first author and research associate in the Stem Cell Potency group at Cambridge.
“But we’ve managed to extract the cells and grow them individually in culture. Naïve stem cells have many potential applications, from regenerative medicine to modeling human disorders.”
Jenny Nichols, PhD, joint senior author of the study, says that one of the most exciting applications of their new technique would be to study disorders that arise from cells that contain an abnormal number of chromosomes. Ordinarily, the body contains 23 pairs of identical chromosomes (22 pairs and one pair of sex chromosomes), but some children are born with additional copies, which can cause problems. For example, children with Down’s syndrome are born with three copies of chromosome 21.
“Even in many ‘normal’ early-stage embryos, we find several cells with an abnormal number of chromosomes,” explains Nichols. “Because we can separate the cells and culture them individually, we could potentially generate ‘healthy’ and ‘affected’ cell lines. This would allow us to generate and compare tissues of two models, one ‘healthy’ and one that is genetically-identical other than the surplus chromosome. This could provide new insights into conditions such as Down’s syndrome.”
The research was supported by the Medical Research Council, Biotechnology and Biological Sciences Research Council, Swiss National Science Foundation, and the Wellcome Trust.
* The drug, MM-401, specifically targets epigenetic chemical markers on histones, the protein “spools” that DNA coils around to create structures called chromatin. These epigenetic changes signal the cell’s DNA-reading machinery and tell it where to start uncoiling the chromatin in order to read it.
A gene called Mll1 is responsible for the addition of these epigenetic changes, which are like small chemical tags called methyl groups. Mll1 plays a key role in the uncontrolled explosion of white blood cells in leukemia, which is why researchers developed the drug MM-401 to interfere with this process. But Mll1 also plays a role in cell development and the formation of blood cells and other cells in later-stage embryos.
Stem cells do not turn on the Mll1 gene until they are more developed. The MM-401 drug blocks Mll1’s normal activity in developing cells so the epigenetic chemical markers are missing. These cells are then unable to continue to develop into different types of specialized cells but are still able to revert to healthy naive pluripotent stem cells.
Abstract of MLL1 Inhibition Reprograms Epiblast Stem Cells to Naive Pluripotency
The interconversion between naive and primed pluripotent states is accompanied by drastic epigenetic rearrangements. However, it is unclear whether intrinsic epigenetic events can drive reprogramming to naive pluripotency or if distinct chromatin states are instead simply a reflection of discrete pluripotent states. Here, we show that blocking histone H3K4 methyltransferase MLL1 activity with the small-molecule inhibitor MM-401 reprograms mouse epiblast stem cells (EpiSCs) to naive pluripotency. This reversion is highly efficient and synchronized, with more than 50% of treated EpiSCs exhibiting features of naive embryonic stem cells (ESCs) within 3 days. Reverted ESCs reactivate the silenced X chromosome and contribute to embryos following blastocyst injection, generating germline-competent chimeras. Importantly, blocking MLL1 leads to global redistribution of H3K4me1 at enhancers and represses lineage determinant factors and EpiSC markers, which indirectly regulate ESC transcription circuitry. These findings show that discrete perturbation of H3K4 methylation is sufficient to drive reprogramming to naive pluripotency.
Abstract of Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass
Conventional generation of stem cells from human blastocysts produces a developmentally advanced, or primed, stage of pluripotency. In vitro resetting to a more naive phenotype has been reported. However, whether the reset culture conditions of selective kinase inhibition can enable capture of naive epiblast cells directly from the embryo has not been determined. Here, we show that in these specific conditions individual inner cell mass cells grow into colonies that may then be expanded over multiple passages while retaining a diploid karyotype and naive properties. The cells express hallmark naive pluripotency factors and additionally display features of mitochondrial respiration, global gene expression, and genome-wide hypomethylation distinct from primed cells. They transition through primed pluripotency into somatic lineage differentiation. Collectively these attributes suggest classification as human naive embryonic stem cells. Human counterparts of canonical mouse embryonic stem cells would argue for conservation in the phased progression of pluripotency in mammals.