See-through ‘MitoFish’ opens a new window on brain diseases
December 6, 2012
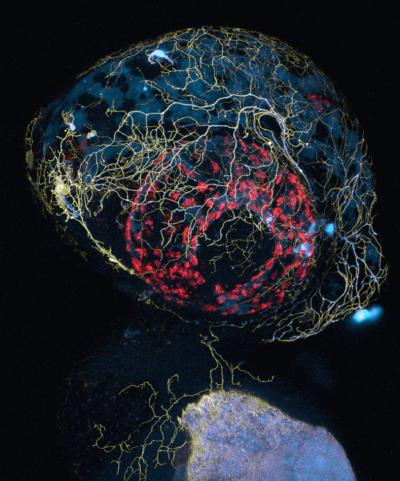
A confocal microscope image of a zebrafish head, showing labeling of sensory axon membranes (yellow), mitochondria (cyan), and autofluorescence (red) (credit: Leanne Godinho and Thomas Misgeld/TU Muenchen)
German scientists have developed a transgenic variety of the zebrafish, which is transparent in the early stages of its life: the “MitoFish,” which enables the scientists to see how brain diseases like Alzheimer’s disturb the transport of mitochondria, the power plants of the cell.
Background
Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, ALS (amyotrophic lateral sclerosis), and MS (multiple sclerosis) are quite different in their effects on patients’ cognitive and motor functions, behavior, and prognosis. Yet on the level of individual neurons, common mechanisms can be observed that either cause or accompany nerve degeneration in a number of different diseases.
One of these is a disturbance in the transport of mitochondria, organelles that play several vital roles in the life of a cell — above all, delivering energy where it is needed. And in a neuron, an extremely power-hungry cell, that means moving mitochondria all the way down its longest extension, the axon.
Studying mitochondria transport in other animal models of neurodegenerative disease, particularly in mice, has been revealing.
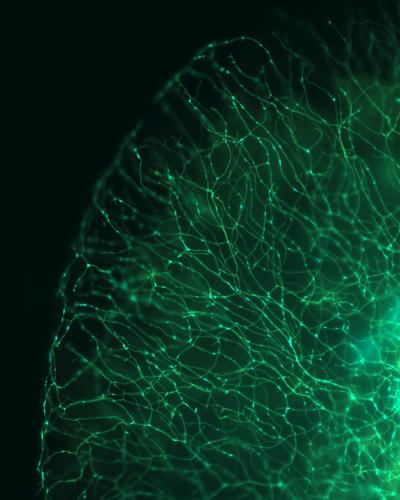
A wide-field image of a zebrafish tail showing labeling of sensory axon membranes (green) and mitochondria (cyan) (credit: Dominik Paquet/LMU Munich)
The MitoFish
But the MitoFish model opens up new possibilities. It was jointly developed in the labs of Prof. Thomas Misgeld of the Technische Universität München (TUM) and Dr. Bettina Schmid, a senior scientist of the German Center for Neurodegenerative Diseases (DZNE) based at the institute of LMU Prof. Christian Haass.
The MitoFish is both readily manipulated, enabling researchers to pose specific questions, and literally transparent — allowing non-invasive in vivo observation of changes relevant to disease processes. It is possible to image a whole, living neuron over time and to follow the movements of mitochondria within it.
“The zebrafish is an established genetic model,” Schmid explains, “which means you can bring foreign genes or certain proteins into a fish to test hypotheses about basic biology, disease mechanisms, or potential therapies.
And because the early embryo is transparent, you can label specific nerve cells with a fluorescent protein and then look at them in an intact, living animal.”
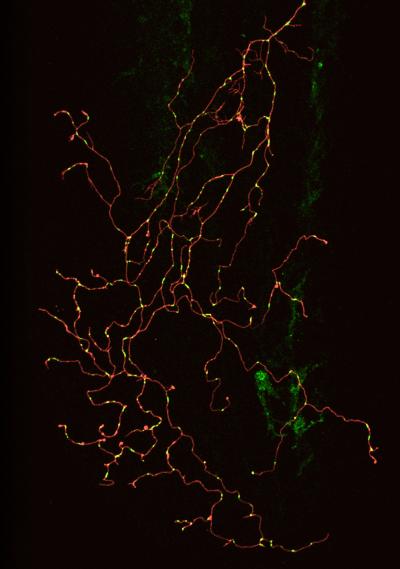
A confocal image of a single zebrafish sensory neuron with labeled membrane (red) and mitochondria (green) (credit: Gabriela Plucinska/TU Muenchen)
This work was supported by the German Research Foundation (DFG); the TUM Institute for Advanced Study, and Excellence Clusters SyNergy (Munich Cluster for Systems Neurology) and CIPSM (Center for Integrated Protein Science Munich); the European Community’s Seventh Framework Programme; and the Alexander von Humboldt Foundation.