Smart skin patch releases blood thinners in closed-loop control system
November 30, 2016
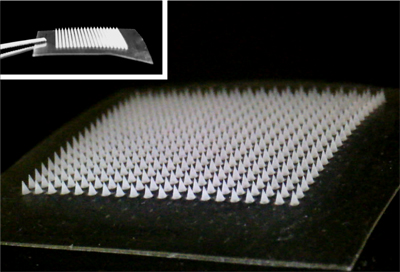
A painless microneedle skin patch for delivering the right amount of a blood-thinning drug (credit: Yuqi Zhang)
North Carolina researchers have developed a smart skin patch designed to monitor a patient’s blood and release a blood-thinning drug, as needed, to prevent thrombosis (dangerous blood clots).
Thrombosis — one of the leading causes of cardiovascular mortalities and morbidities worldwide — occurs when blood clots disrupt the normal flow of blood in the body, which can cause severe health problems such as pulmonary embolism, heart attack, or stroke.
Current treatments often rely on the use of blood thinners, such as heparin, requiring patients to test their blood on a regular basis to ensure proper dosages. Dosage is iffy; too large a dose can cause problems such as spontaneous hemorrhaging, while doses that are too small may not prevent thrombosis.
So researchers at North Carolina State University and the University of North Carolina at Chapel Hill developed a feedback-controlled anti-coagulant smart skin patch to release just the needed level of heparin.
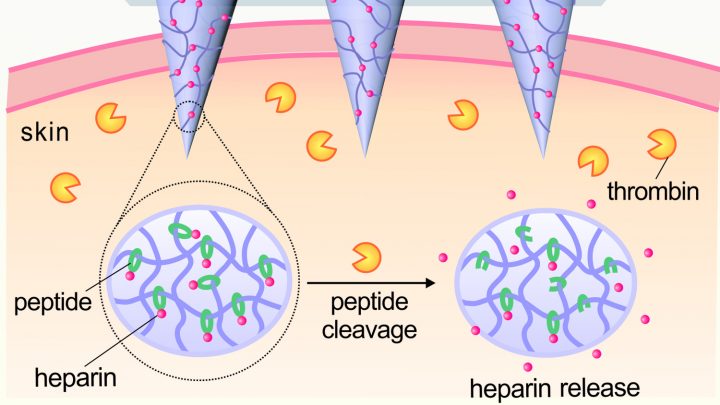
Schematic of skin patch that releases blood-thining heparin (to prevent blood clots) in response to thrombin (an enzyme that initiates clotting in the blood) (credit: Yuqi Zhang)
The patch incorporates an array of painless microneedles made of a material that is responsive to thrombin (an enzyme that initiates clotting in the blood). When elevated levels of thrombin enzymes in the bloodstream come into contact with the microneedle, the enzymes cause the thrombin-responsive material in the microneedles to release the right amount (and no more) of blood-thining heparin into the bloodstream to prevent blood clots.*
The patch was successfully tested in a mouse model.
“Our goal was to generate a patch that can monitor a patient’s blood and release additional drugs when necessary — effectively, a self-regulating system,” says Zhen Gu, co-corresponding author on a paper published Nov. 25, 2016 in Advanced Materials describing the work. Gu is an associate professor in the joint biomedical engineering program at NC State and UNC.
“This paper represents a good first step, and we’re now looking for funding to perform additional preclinical testing,” Gu said.
The work was supported by the Alfred P. Sloan Foundation, NIH’s Clinical and Translational Science Awards, and the National Science Foundation.
* Closed-loop heparin delivery system
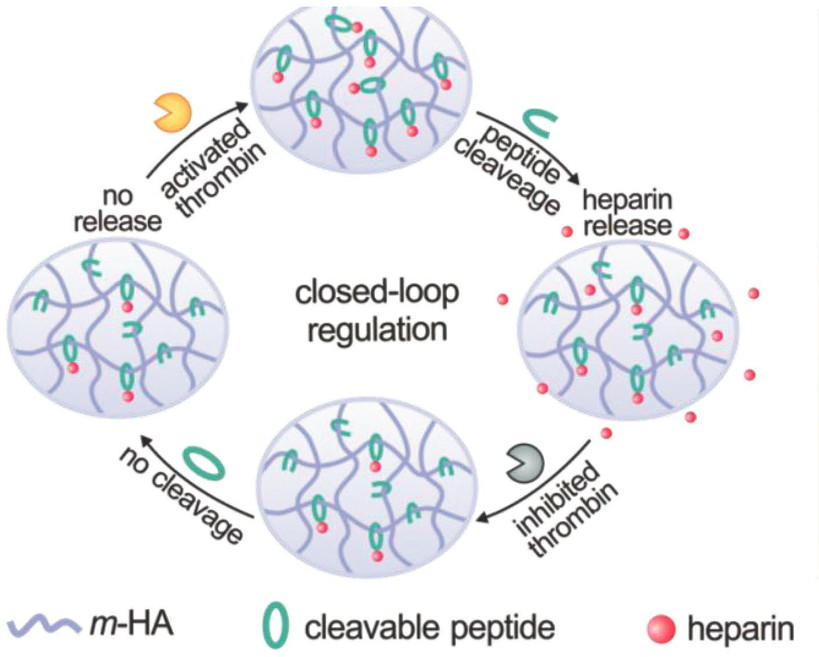
A peptide is introduced as a linker during the conjugation of Heparin to the main chain of hyaluronic acid (which has been UV-modified to be sensitive to thrombin. The presence of thrombin (top) causes the peptide to be cleaved (cut), triggering heparin drug release (right). The released heparin then inhibits the coagulation activation by inactivating thrombin (bottom). In a feedback loop, that process then suppresses further release of heparin (left), minimizing the risk of undesirable spontaneous hemorrhage. (credit: Yuqi Zhang et al./Advanced Materials)
Abstract of Thrombin-Responsive Transcutaneous Patch for Auto-Anticoagulant Regulation
A thrombin-responsive closed-loop patch is developed for prolonged heparin delivery in a feedback-controlled manner. This microneedle-based patch can sense activated thrombin and subsequently releases heparin to prevent coagulation in the blood flow. This “smart” heparin patch can be transcutaneously inserted into skin without drug leakage and can sustainably regulate blood coagulation in response to thrombin.