Surprising discovery of highly dynamic changes in olfactory region of the adult mouse brain
July 5, 2016
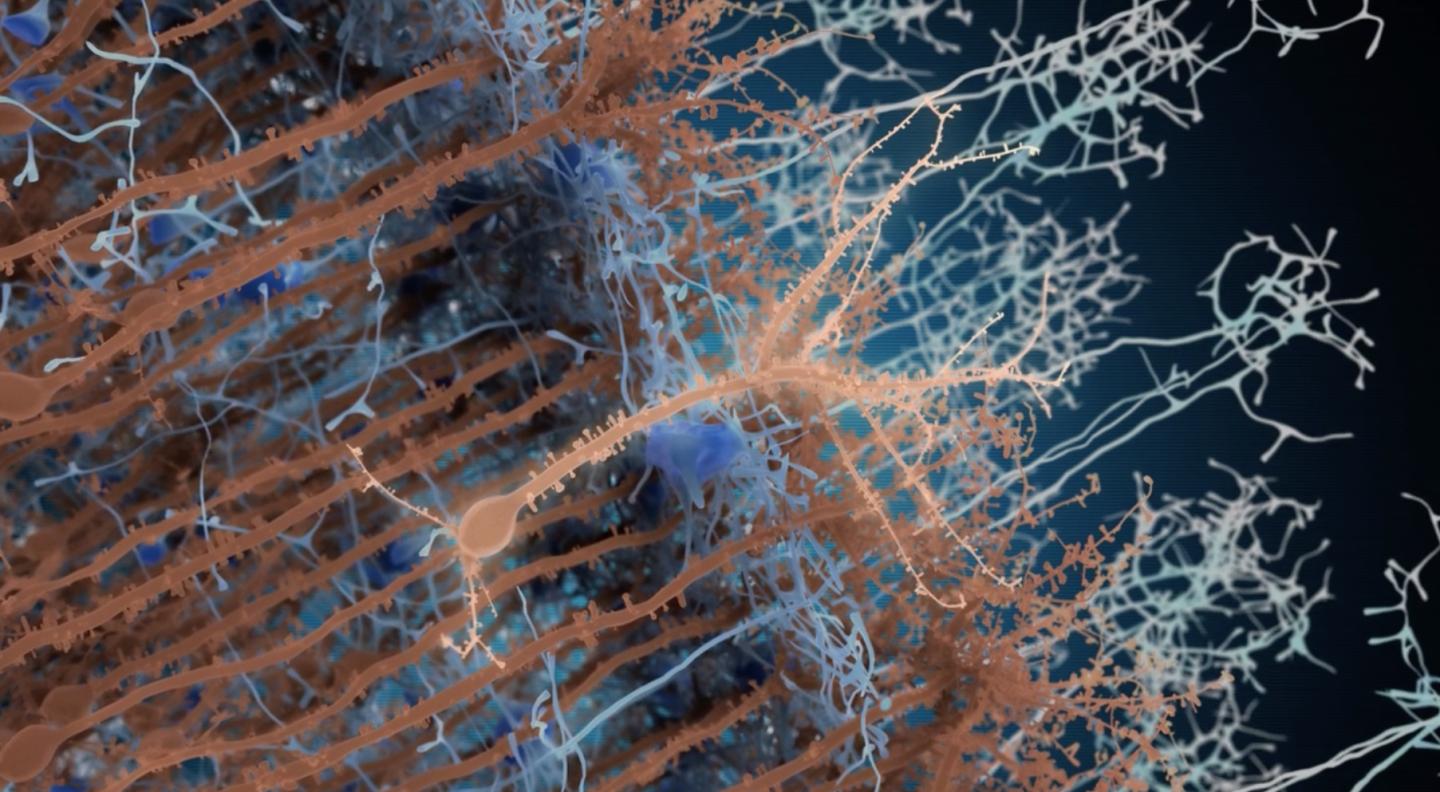
In light brown, in the center of the image, a new adult-born neuron. The neurons in blue are synaptic partner neurons, which connect to the new neurons. The neurons in dark brown are pre-existing neurons. (credit: Institut Pasteur/PM Lledo)
Scientists from the Institut Pasteur and the CNRS have made the surprising discovery that new neurons formed in the olfactory bulb of adult mice are constantly reorganizing the billions of synaptic contacts they establish among themselves (also described as constant structural plasticity).
The researchers found this puzzling because constant structural plasticity is normally confined to specific critical periods after birth, and “plasticity in neural circuits must strike a balance between flexibility and stability so new information can be acquired while previously learned information can be retained,” they note in a paper published in the journal Neuron.
The olfactory bulb in rodents and the hippocampus in humans are two of the areas of the brain capable of constantly regenerating their neurons in adulthood, but it had not been known that olfactory neurons were able to reorganize synaptic contacts.
Watching the brain change through a porthole
To observe the ongoing formation of neuronal circuits, the scientists marked the new granule cell (GC) inter-neurons with a green fluorescent protein (GFP), to view the dynamic changes, using two-photon microscopy imaging via a cranial window.
These experiments were carried out over a period of several months, following the entire life cycle of the new neurons. In the first three weeks of their life, these new neurons extended their cellular projections, known as dendrites, to form several ramifications, which subsequently became very stable.
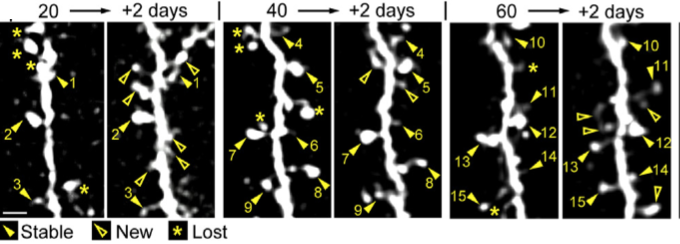
Sample images of the same adult-born granule cell (GC) inter-neuron dendritic segment at a 2-day interval for 20–22, 40–42, and 60–62 days after virus injection (for monitoring) showing stable (closed arrowheads with numbers indexing stable spines), new (open arrowheads), and lost (asterisks) spines (credit: Kurt A. Sailor et al./Neuron)
They next observed neuronal spines — the structure where synapses form — and demonstrated that 20% of the synapses between new and pre-existing neurons were changed on a daily basis — a phenomenon that was also observed in their synaptic partners, the principal olfactory bulb neurons.
Adjusting efficiently and reliably to ongoing sensory changes
Using computer-based models, the authors showed that these dynamics enabled the synaptic network to adjust efficiently and reliably to ongoing sensory changes in the environment, enabling optimal processing of sensory information by the olfactory bulb.
“Our findings suggest that the plasticity of this constantly regenerating region of the brain occurs with continuous physical formation and elimination of synaptic connections. This structural plasticity reveals a unique dynamic mechanism that is vital for the regeneration and integration of new neurons within the adult brain circuit,” concluded the scientists.
More generally, they said, this study suggests a universal plasticity mechanism in brain regions that is closely associated with memory and learning.
This research was supported by the Institut Pasteur and the CNRS and was funded by AG2R-La Mondiale, the French National Research Agency, the “Revive” LabEx, and the “Biopsy” LabEx.
Abstract of Persistent Structural Plasticity Optimizes Sensory Information Processing in the Olfactory Bulb
In the mammalian brain, the anatomical structure of neural circuits changes little during adulthood. As a result, adult learning and memory are thought to result from specific changes in synaptic strength. A possible exception is the olfactory bulb (OB), where activity guides interneuron turnover throughout adulthood. These adult-born granule cell (GC) interneurons form new GABAergic synapses that have little synaptic strength plasticity. In the face of persistent neuronal and synaptic turnover, how does the OB balance flexibility, as is required for adapting to changing sensory environments, with perceptual stability? Here we show that high dendritic spine turnover is a universal feature of GCs, regardless of their developmental origin and age. We find matching dynamics among postsynaptic sites on the principal neurons receiving the new synaptic inputs. We further demonstrate in silico that this coordinated structural plasticity is consistent with stable, yet flexible, decorrelated sensory representations. Together, our study reveals that persistent, coordinated synaptic structural plasticity between interneurons and principal neurons is a major mode of functional plasticity in the OB.