Tissue regeneration using anti-inflammatory nanomolecules
August 28, 2014
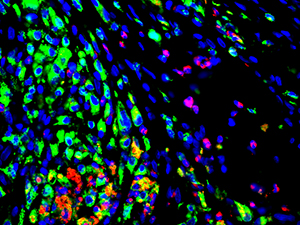
Innate immune cell distribution in regenerating bladder tissue, showing elevated levels of CD68+ macrophages (green) and MPO+ neutrophils (red) (credit: Stanley Manne Children’s Research Institute)
The research group of Arun K. Sharma*, PhD has developed a system for patients with urinary bladder dysfunction that may protect them against an inflammatory reaction** resulting from tissue regeneration, which can negatively impact tissue growth, development and function.
The researchers treated a highly pro-inflammatory biologic scaffold with anti-inflammatory peptide amphiphiles (AIF-PAs). (Self-assembling peptide amphiphiles, or PAs, are biocompatible and biodegradable nanomaterials used in a wide range of settings and applications.) When compared with control PAs, the treated scaffold reduced the innate inflammatory response, resulting in superior bladder function.
This work is published in the journal Biomaterials. “Our findings are very relevant not just for bladder regeneration but for other types of tissue regeneration where foreign materials are utilized for structural support,” says Sharma. These nanomolecules could also be used for treating a “wide range of dysfunctional inflammatory based conditions.”
“This methodology is unique in that it delivers a concentrated ‘dose’ of anti-inflammatory agents to a localized area as opposed to systemic routes utilized by other therapies,” Sharma explained to KurzweilAI in an email. He also said further testing is required, so commercial availability of this product would be “several years away.”
The research team includes members of the Departments of Urology and Medicine at the Feinberg School; Institute for BioNanotechnology in Medicine and the Departments of Biomedical Engineering, Materials Science and Engineering, and Chemical and Biological Engineering at Northwestern University, and the Department of Urology at Loyola University Health System.
This work was performed in collaboration with the Stupp Laboratory at the Institute for BioNanotechnology in Medicine.
*Arun K. Sharma, PhD is Director of Pediatric Urological Regenerative Medicine at Ann & Robert H. Lurie Children’s Hospital of Chicago; Director of Surgical Research at Stanley Manne Children’s Research Institute; Assistant Professor in the Departments of Urology and Biomedical Engineering at Northwestern University Feinberg School of Medicine and Northwestern University; and a member of the Developmental Biology Program of the research institute. The Sharma Group has been working on innovative approaches to tissue regeneration in order to improve the lives of patients. Among their breakthroughs: a medical model for regenerating bladders using stem cells harvested from a donor’s own bone marrow, reported in the Proceedings of the National Academy of Science.
** Anyone who has suffered an injury can probably remember the after-effects, including pain, swelling or redness. These are signs that the body is fighting back against the injury. When tissue in the body is damaged, biological programs are activated to aid in tissue regeneration. An inflammatory response acts as a protective mechanism to enable repair and regeneration, helping the body to heal after injuries such as wounds and burns. However, the same mechanism may interfere with healing in situations in which foreign material is introduced, for example when synthetics are grafted to skin for dermal repair. In such cases, the inflammation may lead to tissue fibrosis, which creates an obstacle to proper physiological function.
Abstract of Biomaterials paper
Current attempts at tissue regeneration utilizing synthetic and decellularized biologic-based materials have typically been met in part by innate immune responses in the form of a robust inflammatory reaction at the site of implantation or grafting. This can ultimately lead to tissue fibrosis with direct negative impact on tissue growth, development, and function. In order to temper the innate inflammatory response, anti-inflammatory signals were incorporated through display on self-assembling peptide nanofibers to promote tissue healing and subsequent graft compliance throughout the regenerative process. Utilizing an established urinary bladder augmentation model, the highly pro-inflammatory biologic scaffold (decellularized small intestinal submucosa) was treated with anti-inflammatory peptide amphiphiles (AIF-PAs) or control peptide amphiphiles and used for augmentation. Significant regenerative advantages of the AIF-PAs were observed including potent angiogenic responses, limited tissue collagen accumulation, and the modulation of macrophage and neutrophil responses in regenerated bladder tissue. Upon further characterization, a reduction in the levels of M2 macrophages was observed, but not in M1 macrophages in control groups, while treatment groups exhibited decreased levels of M1 macrophages and stabilized levels of M2 macrophages. Pro-inflammatory cytokine production was decreased while anti-inflammatory cytokines were up-regulated in treatment groups. This resulted in far fewer incidences of tissue granuloma and bladder stone formation. Finally, functional urinary bladder testing revealed greater bladder compliance and similar capacities in groups treated with AIF-PAs. Data demonstrate that AIF-PAs can alleviate galvanic innate immune responses and provide a highly conducive regenerative milieu that may be applicable in a variety of clinical settings.
Abstract of Proceedings of the National Academy of Sciences paper
Spina bifida (SB) patients afflicted with myelomeningocele typically possess a neurogenic urinary bladder and exhibit varying degrees of bladder dysfunction. Although surgical intervention in the form of enterocystoplasty is the current standard of care in which to remedy the neurogenic bladder, it is still a stop-gap measure and is associated with many complications due to the use of bowel as a source of replacement tissue. Contemporary bladder tissue engineering strategies lack the ability to reform bladder smooth muscle, vasculature, and promote peripheral nerve tissue growth when using autologous populations of cells. Within the context of this study, we demonstrate the role of two specific populations of bone marrow (BM) stem/progenitor cells used in combination with a synthetic elastomeric scaffold that provides a unique and alternative means to current bladder regeneration approaches. In vitro differentiation, gene expression, and proliferation are similar among donor mesenchymal stem cells (MSCs), whereas poly(1,8-octanediol-cocitrate) scaffolds seeded with SB BM MSCs perform analogously to control counterparts with regard to bladder smooth muscle wall formation in vivo. SB CD34+ hematopoietic stem/progenitor cells cotransplanted with donor-matched MSCs cause a dramatic increase in tissue vascularization as well as an induction of peripheral nerve growth in grafted areas compared with samples not seeded with hematopoietic stem/progenitor cells. Finally, MSC/CD34+ grafts provided the impetus for rapid urothelium regeneration. Data suggest that autologous BM stem/progenitor cells may be used as alternate, nonpathogenic cell sources for SB patient-specific bladder tissue regeneration in lieu of current enterocystoplasty procedures and have implications for other bladder regenerative therapies.