X-ray imaging protein molecules at atomic resolution using a graphene cage
February 10, 2014
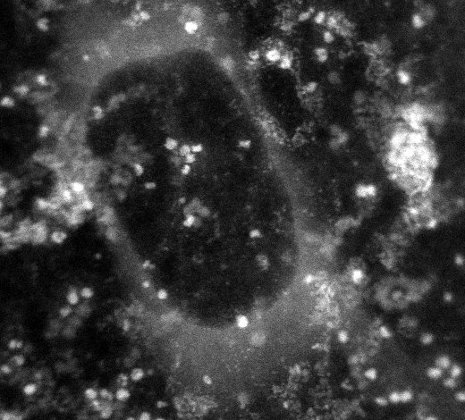
In this image generated by a transmission electron microscope, the white dots are the protein ferritin. The dark circle in the middle is a bubble of liquid trapped within the graphene capsule enclosing the sample. (Credit: MTU)
Michigan Technological University researchers have developed a method of achieving transmission electron microscopy (TEM) atomic-resolution images and nanometer-resolution spectroscopy of biological samples by encapsulating them between two layers of graphene.
The method overcomes the limitations* of TEM imaging of biological samples, and uses a low-dose-rate X-ray imaging technique, so electron beam radiation damage can be reduced to hydrogen-bond-breakage level.
The researchers tried their technique on a biochemical called ferritin that plays a major role in human health.
“It’s a protein that stores and releases iron, which is critical for many body functions, and if ferritin isn’t working right, it may be contributing to lots of diseases, including Alzheimer’s and cancer,” said Tolou Shokuhfar, an assistant professor of mechanical engineering-engineering mechanics.
The team made a microscopic “sandwich,” with ferritin immersed in water as the “filling” and graphene as the “bread,” and sealed the edges, forming “graphene liquid cells.” Then, using a scanning transmission electron microscope, they captured a variety of images showing ferritin’s atomic structure.
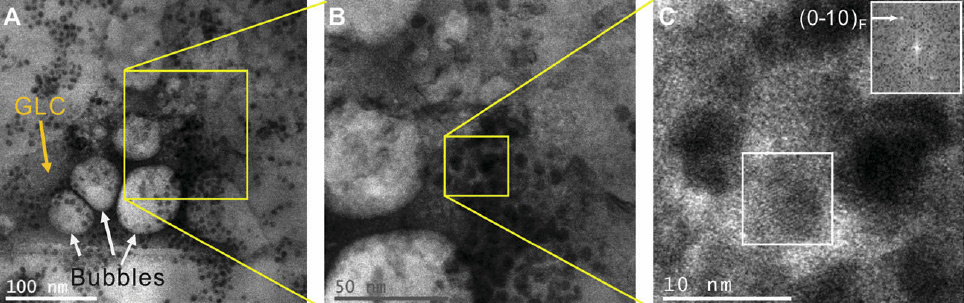
Scanning transmission electron microscope images of ferritin in graphene liquid cells (GLCs) (the bubbles were formed to confirm the presence of liquid). Lattice fringes of the ferritin iron core are resolved in (C) with a spacing of 2.7 Å (.27 nanometers). (Credit: Canhui Wang et al./Advanced Materials)
In addition, they used a special type of X-ray spectroscopy (called Electron Energy-Loss Spectroscopy, or EELS) to identify various atomic and electronic structures within the ferritin. Those images showed that the ferritin was releasing iron and pinpointed its specific form.
If the technique were used to compare ferritin taken from diseased tissue with healthy ferritin, it could provide new insights into illness at the molecular level. Those discoveries could lead to new treatments. “I believe this will allow us to identify disease signatures in ferritin and many other proteins,” Shokuhfar said.
The work was funded by Michigan Technological University and the National Science Foundation. The research was conducted at the University of Illinois-Chicago.
* According the Advanced Materials paper authors, TEM imaging operates in a vacuum, and requires hydrated samples (containing water) to be frozen (at liquid-helium temperature) into ice and then cut into sections, severely altering the material. In addition, the electron beam causes radiation damage, further altering the chemical and physical structure of the sample and limiting spatial resolution. There are liquid-cell designs that use electron transparent silicon nitride membranes, but they do not allow for atomic-resolution imaging or spectroscopy.
Abstract of Advanced Materials paper
Atomic and electronic structures of hydrated ferritin are characterized using electron microscopy and spectroscopy through encapsulation in single layer graphene in a biocompatible manner. Graphene’s ability to reduce radiation damage levels to hydrogen bond breakage is demonstrated. A reduction of iron valence from 3+ to 2+ is measured at nanometer-resolution in ferritin, showing initial stages of iron release by ferritin.