3D-printing a structure with active chemistry
April 4, 2016
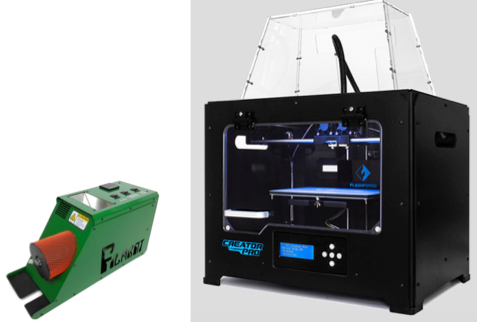
A Filabot Wee Extruder (left) extruded standard ABS filament containing titanium dioxide nanoparticles. The filament was used in a Flashforge Creator Pro 3D Printer (right) to 3D-print a small sponge-like material for mitigating pollution. (credit: Filabot and Zhejiang Flashforge 3D Technology Co., Ltd.)
Researchers at American University have demonstrated the first use of commercial 3D printers to create a structure with active chemistry — in this case, a structure that acts to mitigate pollution.
The researchers added titanium dioxide nanoparticles to standard ABS filament material (used in 3D printers) and extruded a filament that they then used to print a small, sponge-like plastic object on a low-cost Flashforge Creator Pro 3D Printer.
To test whether or not the nanoparticles would remain active after printing, they placed the object in water and added an organic molecule (a pollutant), which was then destroyed. In another test, the titanium dioxide nanoparticles photocatalyzed the degradation of rhodamine 6G (a flourescent tracer dye) in solution.
The researchers next plan to 3D-print a variety of different geometries to determine an optimal printed shape for applications that involve photocatalytic removal of environmental pollutants.
The study, led by chemistry professor Matthew Hartings, was described in an online, open-access paper April 1 in Science and Technology of Advanced Materials.
Abstract of The chemical, mechanical, and physical properties of 3D printed materials composed of TiO2-ABS nanocomposites
To expand the chemical capabilities of 3D printed structures generated from commercial thermoplastic printers, we have produced and printed polymer filaments that contain inorganic nanoparticles. TiO2 was dispersed into acrylonitrile butadiene styrene (ABS) and extruded into filaments with 1.75 mm diameters. We produced filaments with TiO2compositions of 1, 5, and 10% (kg/kg) and printed structures using a commercial 3D printer. Our experiments suggest that ABS undergoes minor degradation in the presence of TiO2 during the different processing steps. The measured mechanical properties (strain and Young’s modulus) for all of the composites are similar to those of structures printed from the pure polymer. TiO2 incorporation at 1% negatively affects the stress at breaking point and the flexural stress. Structures produced from the 5 and 10% nanocomposites display a higher breaking point stress than those printed from the pure polymer. TiO2within the printed matrix was able to quench the intrinsic fluorescence of the polymer. TiO2 was also able to photocatalyze the degradation of a rhodamine 6G in solution. These experiments display chemical reactivity in nanocomposites that are printed using commercial 3D printers, and we expect that our methodology will help to inform others who seek to incorporate catalytic nanoparticles in 3D printed structures.