Mayo Clinic researchers discover drug combination that helps immune system attack cancer cells
July 15, 2016
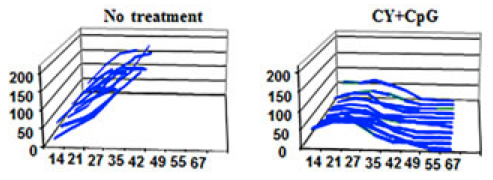
Effects of combination drug treatment on tumor size in square millimeters over 67 days for multiple mice (credit: Soraya Zorro Manrique et al./Oncotarget)
Mayo Clinic researchers have developed a drug combination that could enhance the immune system’s ability to attack cancer cells. The drugs have shown a pronounced therapeutic effect against advanced and metastatic cancers in mice, according to a study published in the July 12 edition of the online journal Oncotarget.
“Cancers can remain inconspicuous in the body for months to years before causing major problems, leading the immune system to coexist rather than to attack cancers,” explains Mayo Clinic cancer immunotherapist Peter Cohen, M.D.
The solution was to combine two drugs: toll-like receptor (TLR) agonists that can mimic invading bacteria (tricking the immune system into attacking cancer as if it were a life-threatening infection) and the chemotherapy agent cyclophosphamide. The combination resulted in permanent eradication of breast and pancreatic cancers, as well as widespread metastases.
The combined weekly treatment was very well tolerated and actually less toxic than either TLR agonists or cyclophosphamide given individually, they also found.
The drug combination also revealed an additional benefit: it activated monocytes (a type of white blood cell) to participate in the killing of cancer cells.
Mayo Clinic is continuing its research in an FDA-approved clinical trial. They are studying whether patients with advanced cancers, including pancreas, breast, colorectal, melanoma and others, respond similarly to mice when cyclophosphamide treatment is paired with the TLR agonist motolimod.
Mayo Clinic | Potential Immunotherapy Drug Combination for Targeting Advanced and Metastatic Cancers
Abstract of Definitive activation of endogenous antitumor immunity by repetitive cycles of cyclophosphamide with interspersed Toll-like receptor agonists
Many cancers both evoke and subvert endogenous anti-tumor immunity. However, immunosuppression can be therapeutically reversed in subsets of cancer patients by treatments such as checkpoint inhibitors or Toll-like receptor agonists (TLRa). Moreover, chemotherapy can leukodeplete immunosuppressive host elements, including myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs). We hypothesized that chemotherapy-induced leukodepletion could be immunopotentiated by co-administering TLRa to emulate a life-threatening infection. Combining CpG (ODN 1826) or CpG+poly(I:C) with cyclophosphamide (CY) resulted in uniquely well-tolerated therapeutic synergy, permanently eradicating advanced mouse tumors including 4T1 (breast), Panc02 (pancreas) and CT26 (colorectal). Definitive treatment required endogenous CD8+ and CD4+ IFNγ-producing T-cells. Tumor-specific IFNγ-producing T-cells persisted during CY-induced leukopenia, whereas Tregs were progressively eliminated, especially intratumorally. Spleen-associated MDSCs were cyclically depleted by CY+TLRa treatment, with residual monocytic MDSCs requiring only continued exposure to CpG or CpG+IFNγ to effectively attack malignant cells while sparing non-transformed cells. Such tumor destruction occurred despite upregulated tumor expression of Programmed Death Ligand-1, but could be blocked by clodronate-loaded liposomes to deplete phagocytic cells or by nitric oxide synthase inhibitors. CY+TLRa also induced tumoricidal myeloid cells in naive mice, indicating that CY+TLRa’s immunomodulatory impacts occurred in the complete absence of tumor-bearing, and that tumor-induced MDSCs were not an essential source of tumoricidal myeloid precursors. Repetitive CY+TLRa can therefore modulate endogenous immunity to eradicate advanced tumors without vaccinations or adoptive T-cell therapy. Human blood monocytes could be rendered similarly tumoricidal during in vitro activation with TLRa+IFNγ, underscoring the potential therapeutic relevance of these mouse tumor studies to cancer patients.